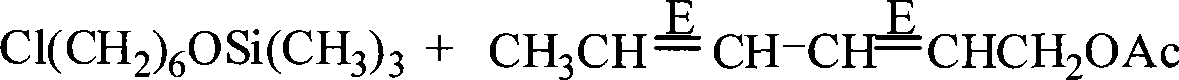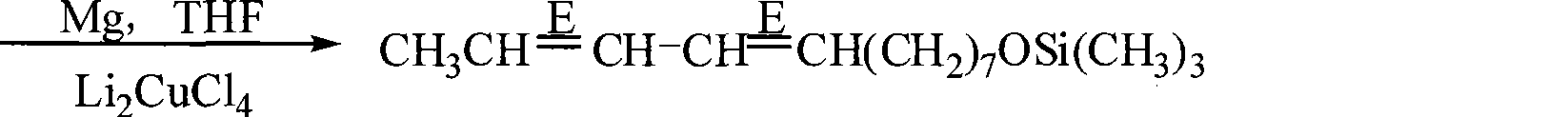Preparation of (8E, 10E)-8,10- dodecadienol-1-alcohol
A technology of dodecadiene and 10-, applied in the field of -8, can solve the problems of difficult industrial production, high price, environmental, human body hazards, etc., and achieves the effects of easy procurement, low price, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation method of (8E, 10E)-8,10-dodecadien-1-ol of the present embodiment has the following steps:
[0022] ① A thermometer, a stirrer, a drier and a dropping funnel are installed on a 500mL reaction flask, and 100g of Cl(CH 2 ) 6 OH (0.73mol) and 78g of Na 2 CO 3 (0.74mol), the pH value of the solution is 9, slowly add 103g of (CH 3 ) 3 SiCl (0.95mol), react at 30°C for 6h after addition. Then add 200ml of water for hydrolysis; extract three times with ether, combine the ether extract, then wash the ether extract with saline and distilled water until neutral, and use CP grade anhydrous Na 2 SO 4 Dry, remove the ether solvent with normal pressure distillation, then collect (130~132) ℃ / 5mmHg cuts with vacuum distillation to obtain 139.7g of chlorohexyloxytrimethylsilane with a purity of 96%, yield up to 92%.
[0023] ②Put 10g of magnesium chips (0.416mol), 0.05g of iodine grains, and 100mL of tetrahydrofuran into a 500mL four-necked bottle, install a sti...
Embodiment 2~ Embodiment 5
[0027] The preparation method of each embodiment is basically the same as that of Example 1, and the differences are shown in Table 1.
[0028] Table 1
[0029]
[0030] In Table 1: raw material A is (2E,4E)-2,4-hexadien-1-ol acetate. Product A is chlorohexyloxytrimethylsilane. Product B is (8E,10E)-8,10-dodecadien-1-oxytrimethylsilane. Product C is (8E,10E)-8,10-dodecadien-1-ol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com