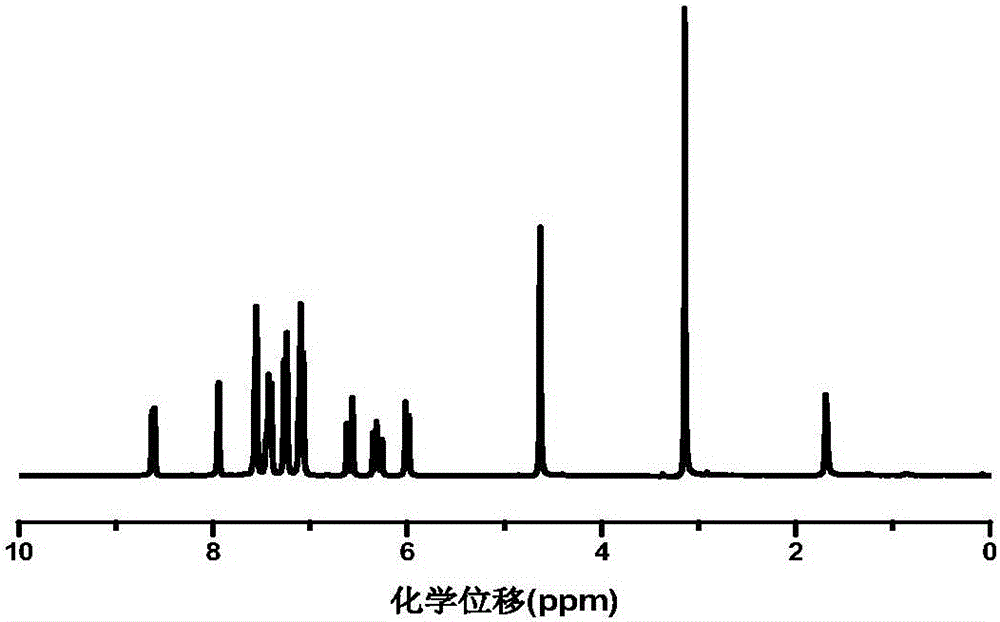
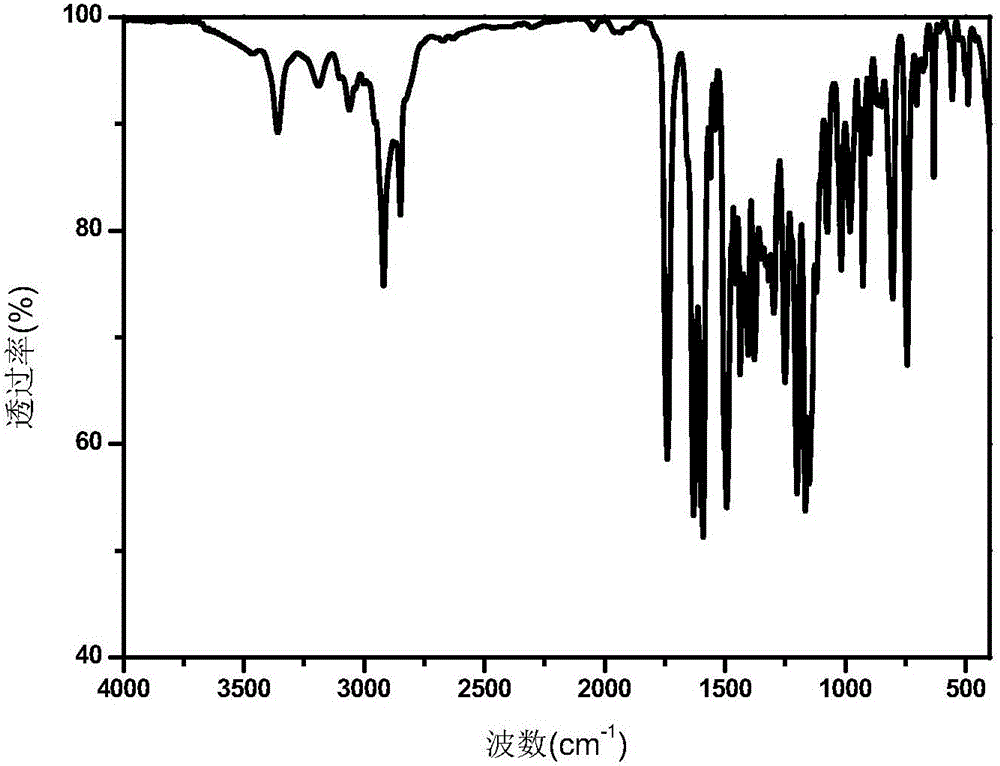
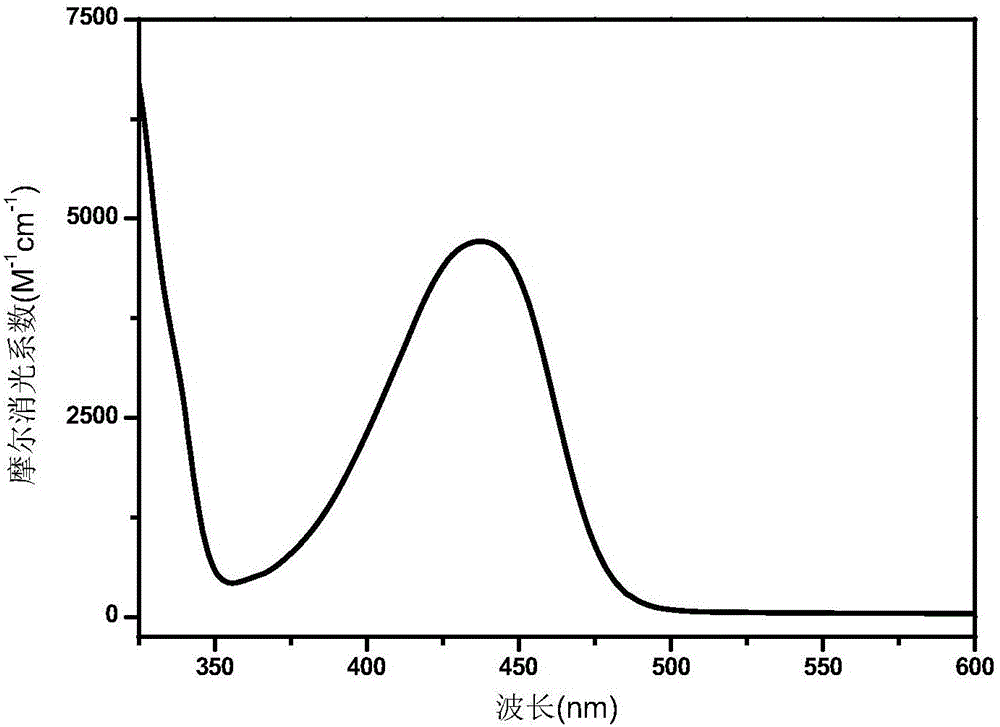
Polymerizable type thioxanthone visible light initiator containing acrylate or methacrylate and preparation method
A technology of methacrylate and thioxanthone is applied in the field of visible light initiator of thioxanthone and preparation thereof, and can solve the problems of unfavorable macromolecular photoinitiator, hindered photoactive group, complicated synthesis method, etc. Wide application prospects, high initiation efficiency, and strong penetrating energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of thioxanthone containing phenolic hydroxyl
[0035] In a 100mL round bottom flask, add 2.41g (10mmol) of 2-methylaminothioxanthone, 2.46g (10mmol) of 4-benzoyloxybenzyl chloride, 2.76g (20mmol) of anhydrous potassium carbonate, potassium iodide 0.1g, 50mL of N,N-dimethylformamide, stirred at room temperature for 10h, a yellow solid precipitated, filtered, the filter cake was introduced into a 100mL round bottom flask, heated to reflux with methanol for 2h, hot filtered, the filter cake was dissolved in tetrahydrofuran, The filtrate was poured into petroleum ether for reprecipitation to obtain 2.74 g of 2-(N-methyl-N-p-hydroxybenzyl)aminothioxanthone with a yield of 79%.
[0036] (2) Preparation of thioxanthone containing acrylate
[0037] Add 0.69 g (2 mmol) of 2-(N-methyl-N-p-hydroxybenzyl) aminothioxanthone into a 100 mL single-necked flask equipped with a stirring bar, seal it with a saline plug, and inject dry Dichloromethane 20mL, anhydrous trie...
Embodiment 2
[0044] (1) Preparation of thioxanthone containing phenolic hydroxyl
[0045] In a 100mL round bottom flask, add 2.83g (10mmol) of 3-methyl-2-isopropylaminothioxanthone, 2.70g (11mmol) of 4-benzoyloxybenzyl chloride, and 2.12g of anhydrous sodium carbonate (20mmol), potassium iodide 0.1g, N,N-dimethylformamide 50mL, stirred at room temperature for 16h, a yellow solid precipitated, filtered, the filter cake was introduced into a 100mL round-bottomed flask, methanol was heated to reflux for 3h, hot filtered, filtered The cake was dissolved in tetrahydrofuran, and the filtrate was poured into petroleum ether for reprecipitation to obtain 2.96 g of 3-methyl-2-[N-isopropyl-N-(4-hydroxybenzyl)]aminothioxanthone with a yield of 76%. .
[0046] (2) Preparation of thioxanthone containing alcoholic hydroxyl
[0047] Add 1.17 g (3 mmol) of 3-methyl-2-[N-isopropyl-N-(4-hydroxybenzyl)]aminothioxanthone to a 100 mL single-necked flask equipped with a stirring bar, 3-chloro 0.31g (3.3mmol)...
Embodiment 3
[0056] (1) Preparation of thioxanthone containing phenolic hydroxyl
[0057] In a 100mL round bottom flask, add 3.39g (10mmol) of 3-ethyl-2-hexylaminothioxanthone, 3.20g (13mmol) of 4-benzoyloxybenzyl chloride, and 2.76g of anhydrous potassium carbonate (20mmol), potassium iodide 0.1g, N,N-dimethylformamide 50mL, stirred at room temperature for 24h, a yellow solid precipitated, filtered, the filter cake was introduced into a 100mL round-bottomed flask, methanol was heated to reflux for 5h, hot filtered, filtered The cake was dissolved in tetrahydrofuran, and the filtrate was poured into petroleum ether for reprecipitation to obtain 3.85 g of 3-ethyl-2-[N-hexyl-N-(4-hydroxybenzyl)]aminothioxanthone with a yield of 87%.
[0058] (2) Preparation of thioxanthone containing alcoholic hydroxyl
[0059] Add 1.33 g (3 mmol) of 3-ethyl-2-[N-hexyl-N-(4-hydroxybenzyl)]aminothioxanthone to a 100 mL single-necked flask equipped with a stirring bar, 6-bromo-n-hexane Alcohol 0.65g (3.6mmol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com