Chiral inducer for synthesizing (1R,2S)-Bedaquiline
A technology of chiral induction and bedaquiline, applied in organic chemistry, organic chemical methods, etc., can solve the problem of low yield of bedaquiline and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
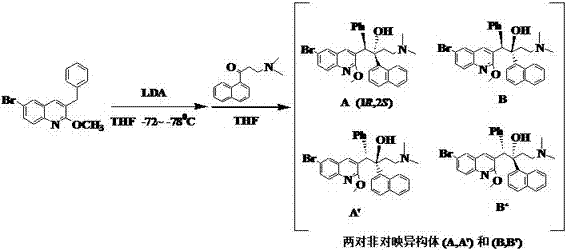
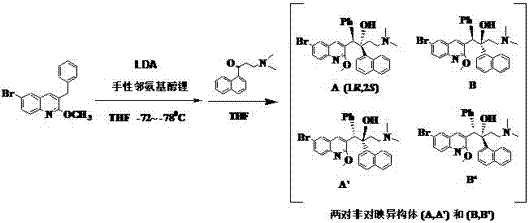
[0033] Under nitrogen protection, add THF 45ml and N-benzyl-L-prolinol 15.4g (80.5mmol, 1.10eq) into a dry 500mL four-necked glass reaction flask, and place the reaction flask in a cold trap (- 72~ -78℃), then add 2.5M n-butyllithium n-hexane solution 32ml (80.5mmol), then add 2.0M LDA (mixed solvent of heptane-ethylbenzene-tetrahydrofuran) 44.0ml (88.0mmol), THF 21 ml. Dissolve 24.0 g (73.2 mmol, 1.0 eq) of 6-bromo-3-benzyl-2-methoxyquinoline in THF 30 ml, slowly drop it into the above-mentioned four-necked bottle, and complete the dropwise addition within 70 min , keep the internal temperature at -72~-78°C during the feeding process, and continue stirring for 3 h after the addition. In the following 160 min, slowly add dropwise a solution of 17.4 g (76.9 mmol) of 3-N,N-dimethylamino-1-naphthyl-1-propanone and 50 ml of THF, and keep the internal temperature during the dropwise addition -72~-78°C, after adding, continue to react for 3h. The HPLC test results of the reaction...
Embodiment 2
[0037] Under nitrogen protection, add THF 67ml and N-benzyl-L-prolinol 22.4g (117mmol, 1.50eq) into a dry 500mL four-necked glass reaction flask, and place the reaction flask in a cold trap (-72 ~ -78°C), then add 46.5ml (117mmol) of 2.5M n-butyllithium n-hexane solution, other steps and material consumption are the same as in Example 1, the obtained reaction material HPLC detection result: enantiomer A / A' =81:19; diastereomer (A+A') / (B+B') = 5:1.
Embodiment 3
[0039] Under nitrogen protection, add THF 90ml and N-benzyl-L-prolinol 30.8g (161mmol, 2.0eq) into a dry 500mL four-necked glass reaction flask, and place the reaction flask in a cold trap (-72 ~ -78°C), and then add 64ml (161mmol) of 2.5M n-butyllithium n-hexane solution, other steps and material consumption are the same as in Example 1, and the obtained reaction material HPLC detection result: enantiomer A / A'=82 :18; diastereomer (A+A') / (B+B') = 5.1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com