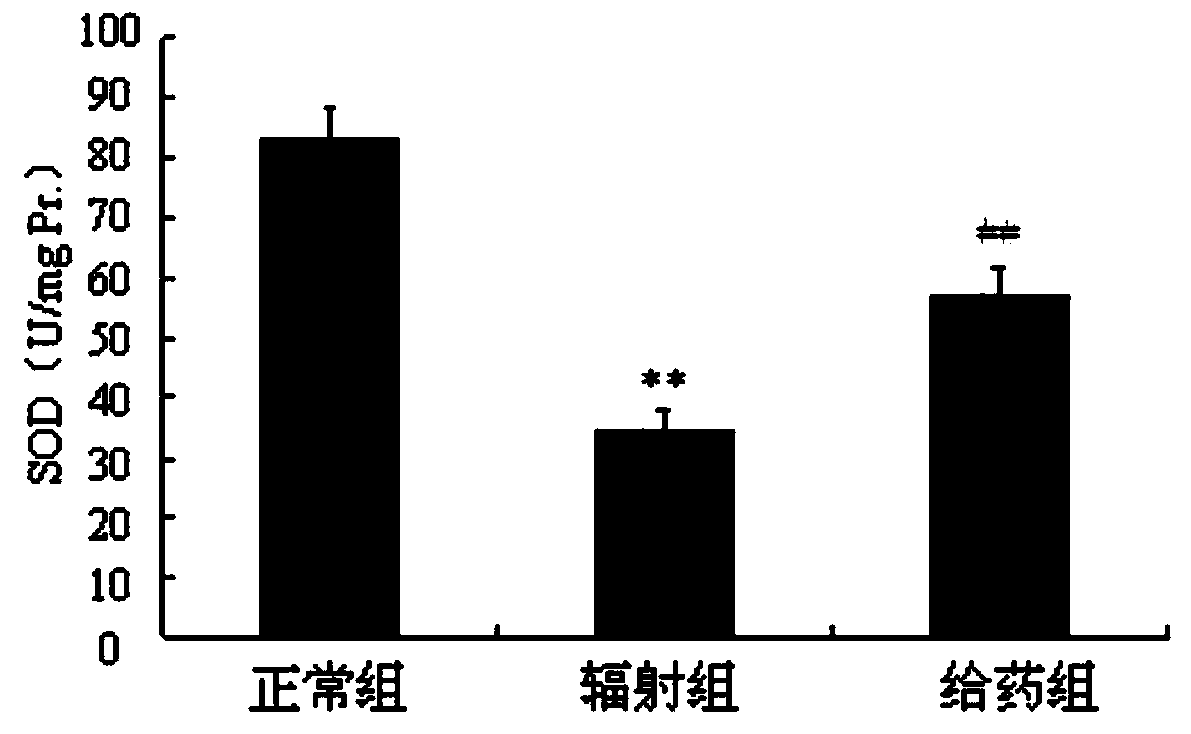
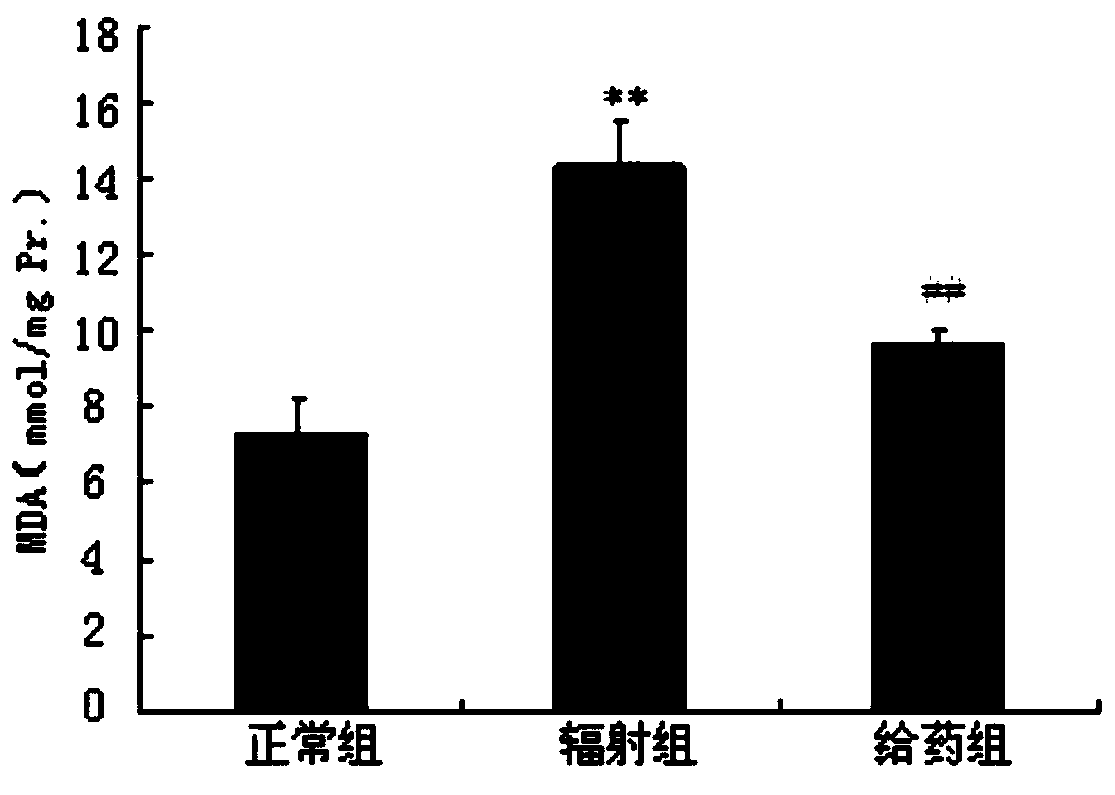
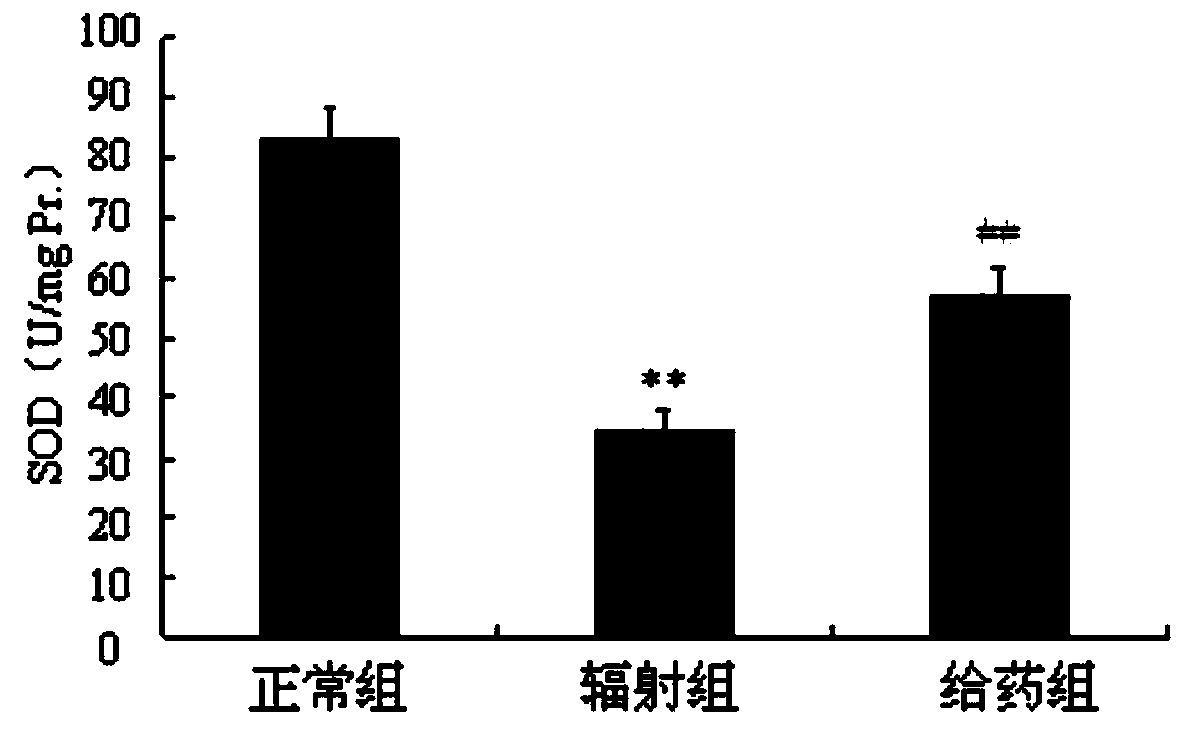
Radiation protection compound as well as synthesis method and application thereof
A technology for radiation protection and synthesis method, applied in the field of medicine, can solve problems such as mitochondrial dysfunction, and achieve the effects of high yield, mild reaction conditions and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The present embodiment has the radioprotective compound of general structural formula (I),
[0040]
[0041] Among them, R 1 ~R 4 , R' 1 ~R' 4 = NO 2 , OH, F, Cl, Br, H;
[0042] The prolinol of general structural formula (I) is L-prolinol.
[0043] The specific process of the synthetic method of radiation protection compound of the present invention is:
[0044] Step 1, the synthesis of intermediate 1
[0045] Weigh 2.62g of triphenylphosphine (TPP, 10mmol) and 2.07g of 6-bromohexanoic acid (10.5mmol), dissolve them in anhydrous acetonitrile, then reflux for 16h under nitrogen protection and stirring, and use TLC to determine whether the reflux At the end of the reaction, the reaction solution obtained by the reflux reaction was placed in a rotary evaporator to spin dry the solvent, and the obtained solid was ground into a powder with a mortar, washed 2 to 3 times with n-hexane and then suction filtered to obtain 2.94 g of a white solid. Intermediate 1, afte...
Embodiment 2
[0055] The difference between this embodiment and Example 1 is that the prolinol in the general structural formula (I) of the radioprotective compound is L-prolinol;
[0056] In the synthesis process of the radiation protection compound, the L-prolinol added in step 2 is replaced by D-prolinol, and the corresponding intermediate 2, intermediate 3 and intermediate 4 obtained are all in D-configuration, and in step 5 The product obtained was TPP-D-NIT-1.
Embodiment 3
[0058] The present embodiment has the radioprotective compound of general structural formula (II),
[0059]
[0060] The prolinol of general structural formula (II) is L-prolinol.
[0061] The specific process of the synthetic method of radiation protection compound of the present invention is:
[0062] Step 1, the synthesis of intermediate 5
[0063] Dissolve 2.62g of triphenylphosphine (TPP, 10mmol) and 7.3g of 1,6-dibromohexane (30mmol) in anhydrous acetonitrile and stir, then reflux for 12h, and use TLC to detect the end point of the reaction; The product system obtained by the reaction was placed in a rotary evaporator to spin dry the solvent, separated by column chromatography to obtain 3.42g light yellow oil, and then purified by column chromatography (eluent was made of dichloromethane and methanol according to the volume of 10:1 ratio prepared) to obtain intermediate 5, after calculation, the yield of intermediate 5 is 80%; the mass spectrometry analysis result H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com