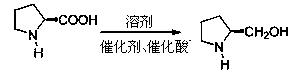
Method for preparing L-prolinol through high-pressure hydrogenization of L-proline
A high-pressure hydrogenation, proline technology, applied in the direction of organic chemistry and the like, can solve the problems of long process route, large pollution, troublesome post-processing, etc., and achieves the effects of high yield, short process route and easy post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 10g of L-proline, 20g of solvent isopropanol, 1g of catalyst 5%Ru / C (self-made 50% moisture), 0.1g of phosphoric acid, controlled pressure at 6-8 MPa, temperature 140-150°C, reduced for 8h, obtained L-prolinol, (sampling analysis GC L-prolinol 95.6%).
Embodiment 2
[0028] 50g of L-proline, 100g of solvent isopropanol, 5g of catalyst 5%Ru / C (self-made 50% moisture), 0.5g of phosphoric acid, controlled pressure at 6-8 MPa, temperature 140-150°C, reduced for 8h, obtained L-prolinol, (sampling analysis GC L-prolinol 95.9%).
Embodiment 3
[0030] L-proline 100g, solvent isopropanol 200g, catalyst 5%Ru / C (self-made 50% moisture) 10g, phosphoric acid 1g, control pressure at 6-8 MPa, temperature 140-150°C, reduce for 8h, get L- Prolinol, (sampling analysis GC L-prolinol 95.7%), add 6g of sodium hydroxide, filter out the catalyst, recover isopropanol, and rectify under reduced pressure to obtain 80.8g of L-prolinol, yield 92% , GC detection content 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com