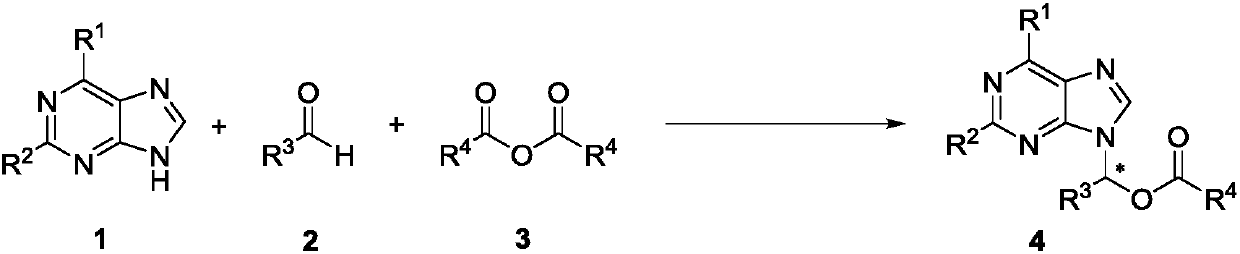
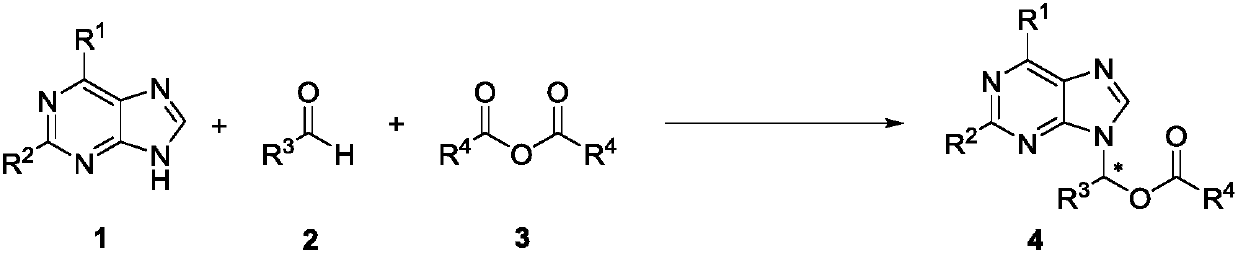
Method for synthesizing chiral purine noncyclic nucleoside through dynamic and kinetic resolution of purine, aldehyde and anhydride
A dynamic kinetics, cyclic nucleoside technology, applied in organic chemistry methods, chemical instruments and methods, asymmetric synthesis, etc., can solve the problems of difficult preparation and high cost of chiral substrates, and achieve a rich and efficient synthesis method of product structure. , the effect of easy availability of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025]
[0026] Reaction conditions: 1a (0.1mmol), catalyst (10mol%), and base (10mol%) were added ina test tube, followed by adding 3a, 2a, and solvent (2mL) at room temperature. b Isolated yield based on 1a. c Determined by chiral HPLC analysis. d At 50℃. MS (60mg) was added.
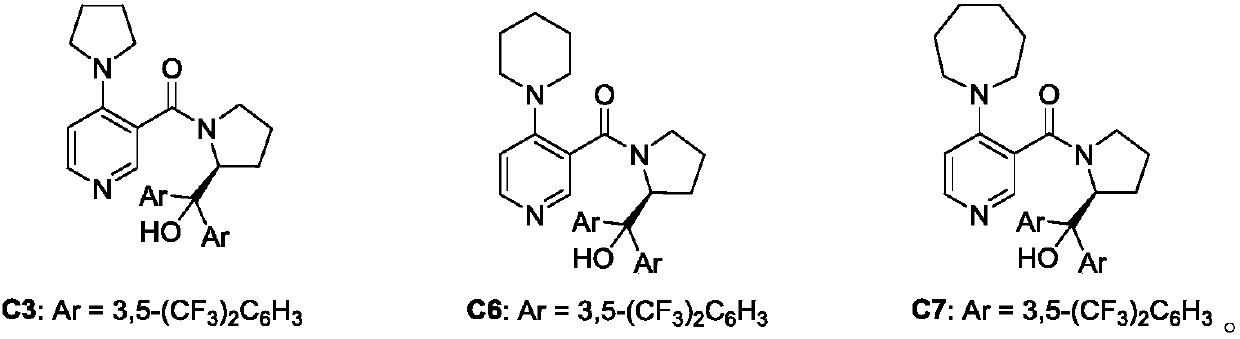
[0027] In the process of screening the reaction conditions, the influence of PPY-type chiral catalysts on the reaction was first investigated (markers 1-7). At the same time, by comparing the effects of different catalysts on the reaction, the best catalyst for catalyst C7 was determined.
[0028] Investigation of reaction conditions: In a 10mL vacuum tube, add 6-chloropurine 1a (15.4mg, 0.1mmol), C7 (7.7mg, 10mol%), sodium carbonate (1mg, 0.10mmol) and acetaldehyde 2a (16μL, 0.3 mmol), acetic anhydride 3a (24 μL, 0.3 mmol). Then add 2mL of toluene, 60mg of Molecular sieve. The reaction tube was sealed, and the reaction tube was placed under a magnetic stirrer at room temperatu...
Embodiment 2
[0041]
[0042] In a 10 mL vacuum tube, add 6-chloropurine 1 (15.4 mg, 0.1 mmol), C7 (7.7 mg, 10 mol%), sodium carbonate (1 mg, 0.10 mmol) and benzaldehyde 2 (30 μL, 0.3 mmol), acetic anhydride 3 (24 μL, 0.3 mmol). Then add 2mL of toluene, 60mg of Molecular sieve. Seal the reaction tube, and place the reaction tube under a magnetic stirrer at room temperature to react for 3 days. The reaction was followed by TLC. After the reaction was terminated, the reaction solution was concentrated in vacuo, and then the target compound 4x was obtained by column chromatography. The yield was 35%, 40% ee.
Embodiment 3
[0044]
[0045] In a 10 mL vacuum tube, add 6-chloropurine 1 (15.4 mg, 0.1 mmol), C7 (7.7 mg, 10 mol%), sodium carbonate (1 mg, 0.10 mmol) and acetaldehyde 2 (16 μL, 0.3 mmol), benzyl Anhydride 3 (60mg, 0.3mmol). Then add 2mL of toluene, 60mg of Molecular sieve. Seal the reaction tube, and place the reaction tube under a magnetic stirrer at room temperature to react for 3 days. The reaction was tracked by TLC. After the reaction was terminated, the reaction solution was concentrated in vacuo, and then the target compound 4y was obtained by column chromatography with a yield of 38%, 36% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com