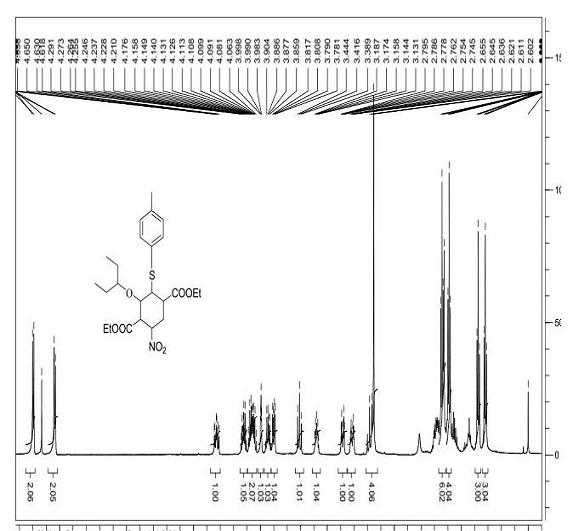
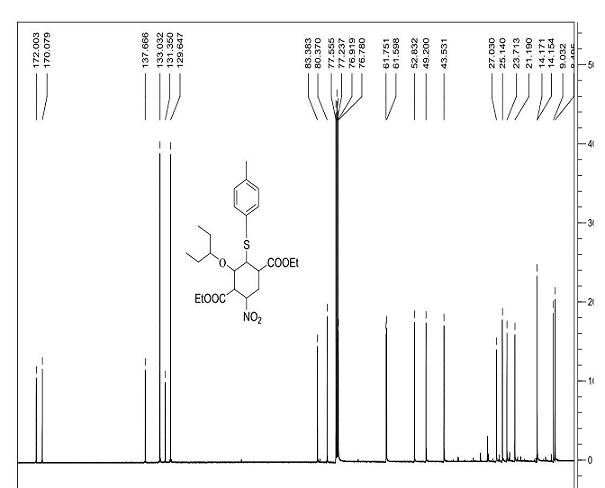
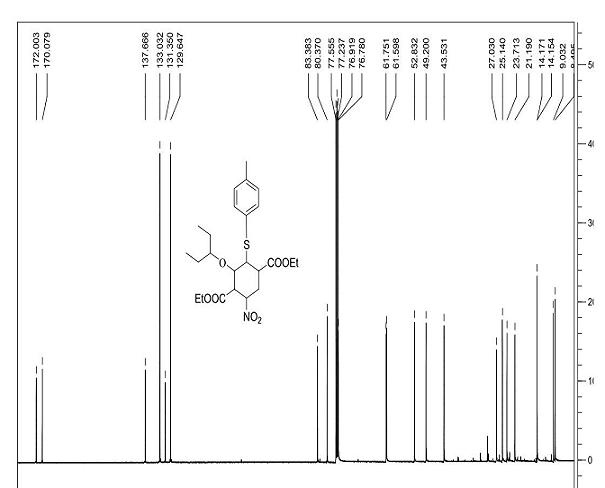
Tamiflu intermediate and synthesis method thereof
A synthetic method and intermediate technology, applied in the field of Tamiflu intermediate and its synthesis, can solve the problems of non-recyclable, high catalyst price, etc., and achieve the effects of good stereoselectivity, high target content and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: See the aforementioned comparative example 2 for the synthesis circuit.
[0032] Step 1): 25 °C, o-nitrobenzoic acid (38.6g, 0.231mol) and (R)-N,N-dimethylbenzylamine prolinol silyl ether (3) (25.4g, 0.058mol) Pre-stir for 30 minutes, add 3-pentoxyacetaldehyde (1) (225g, 1.73 mol) and 3-nitroacrylate (2) (168g, 1.16mol) in dichloromethane (2L) after salt formation After the reactants were stirred at 25 °C for 24 hours, the solvent was removed under reduced pressure, and the n-hexane (2L) was dissolved and filtered. The solids could be used for the catalytic reaction again, and the filtrate containing product (4) was directly used in the next reaction.
[0033] Step 2): Add 2-(diethoxy, phosphoryl) ethyl acrylate (5) (409.2g, 1.73mol) and cesium carbonate (1.13Kg, 3.47mol) to the filtrate at 0°C and keep it at 0°C Stir for 3h, remove the solvent under reduced pressure, add ethanol (3L) to the above crude product, and stir for 15min at 25°C.
[0034] Step 3): Add p-...
Embodiment 2
[0036] Embodiment 2: The synthesis circuit is as follows,
[0037]
[0038] Step 1): 25 °C, pre-stir chloroacetic acid (21.8g, 0.231mol) and (R)-N,N-dimethylbenzylamine prolinol (3) (25.4g, 0.058mol) for 30min After salt formation, add 3-pentoxyacetaldehyde (1) (225g, 1.73 mol) and 3-nitroacrylate ethyl (2) (168g, 1.16mol) in dichloromethane (2L) solution, the reactant After stirring for 40 min at 25 °C, the solvent was removed under reduced pressure, and the n-hexane (2L) was dissolved and filtered. The solids could be used for the catalytic reaction again, and the filtrate containing product (4) was directly used in the next reaction.
[0039] Step 2): Add 2-(diethoxy, phosphoryl) ethyl acrylate (5) (409.2g, 1.73mol) and cesium carbonate (1.13Kg, 3.47mol) to the filtrate at 0°C. Stir for 3h at C, remove the solvent under reduced pressure, add ethanol (3L) to the above crude product, and stir for 15min at 25°C.
[0040] Step 3): Add p-methylthiophenol (716.7g, 5.78mol) at -15°C an...
Embodiment 3
[0042] Embodiment 3: The synthesis circuit is as follows,
[0043]
[0044] Step 1): 25 °C, pre-stir chloroacetic acid (21.8g, 0.231mol) and (R)-N,N-dimethylbenzylamine prolinol (3) (35.1g, 0.058mol) for 30 Minutes, after salt formation, add 3-pentoxyacetaldehyde (1) (225g, 1.73 mol) and ethyl 3-nitroacrylate (2) (168g, 1.16mol) in dichloromethane (2L) solution, react After stirring for 40 minutes at 25 °C, the solvent was removed under reduced pressure. After dissolving in n-hexane (2L), it was filtered. The solids could be used for catalytic reaction again, and the filtrate containing product (4) was directly used in the next reaction.
[0045] Step 2): Add 2-(diethoxy, phosphoryl) ethyl acrylate (5) (409.2g, 1.73mol) and cesium carbonate (1.13Kg, 3.47mol) to the filtrate at 0°C and keep it at 0°C Stir for 3h, remove the solvent under reduced pressure, add ethanol (3L) to the above crude product, and stir for 15min at 25°C.
[0046] Step 3): Add p-methylthiophenol (716.7g, 5.78mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com