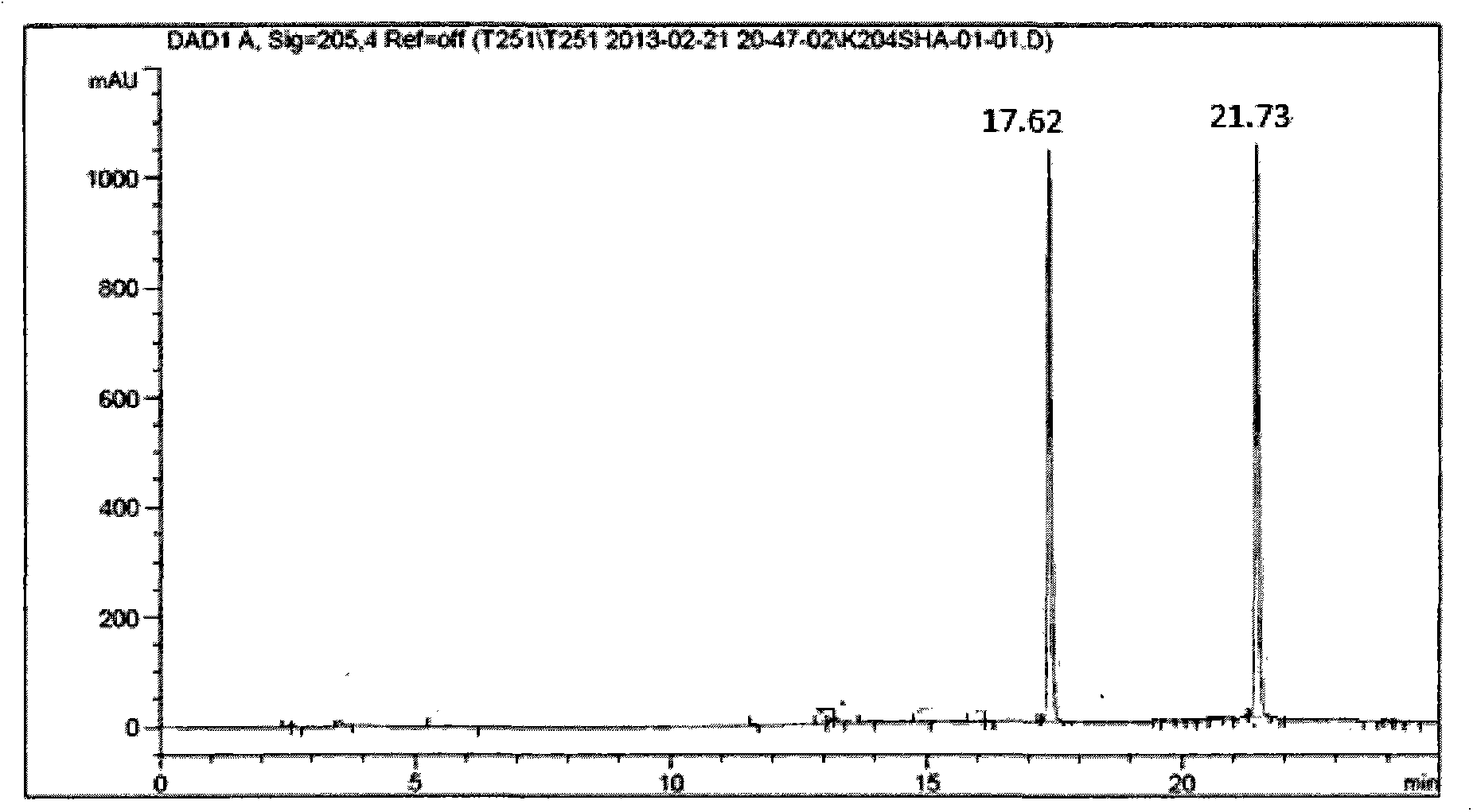
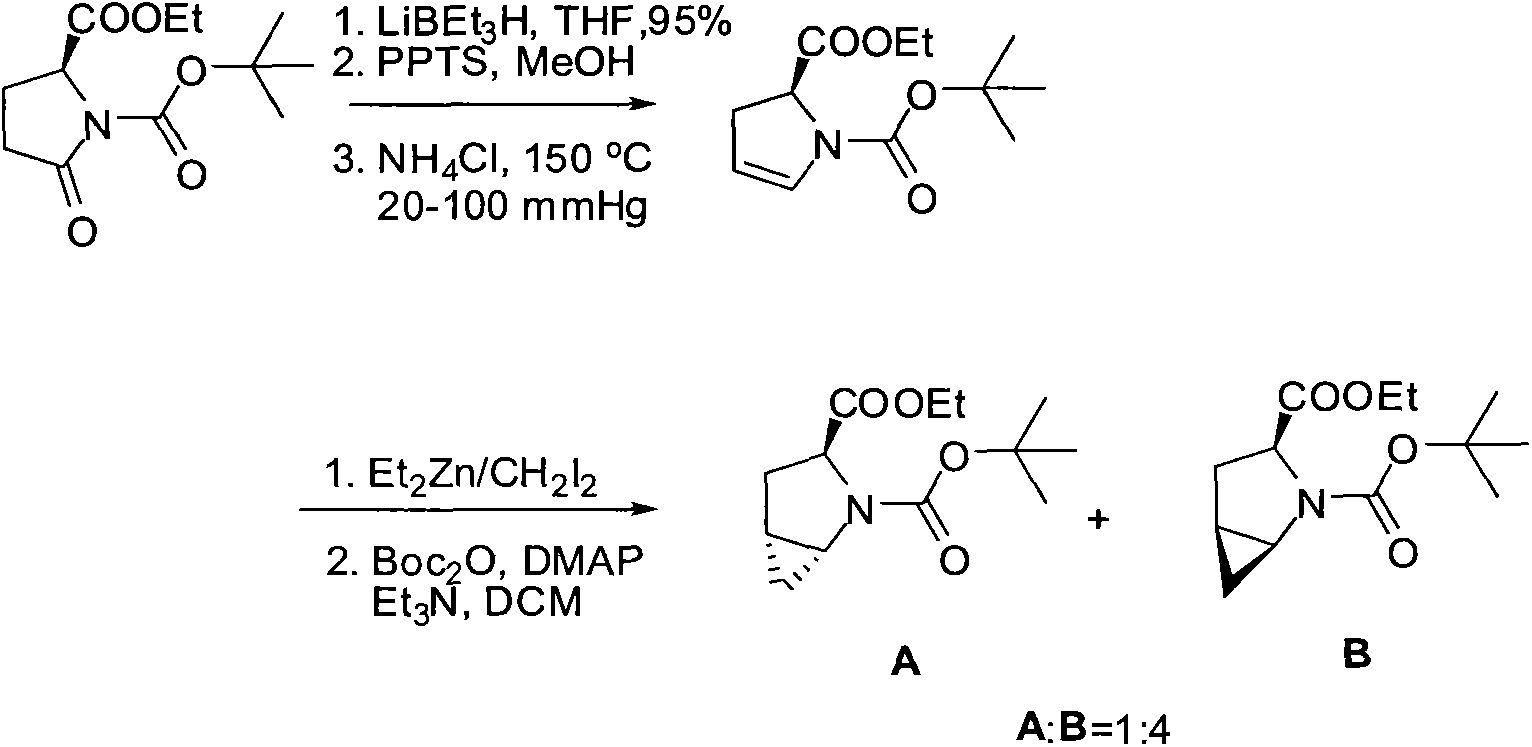
R-diphenyl prolinol chiral organic small molecule compound with cyclopropane structure and synthesis method of R-diphenyl prolinol chiral organic small molecule compound
A technology for diphenylprolinol and small molecular compounds, applied in the field of compound preparation, can solve problems such as poor enantioselectivity and diastereoselectivity, and achieve the effects of simple operation, high catalytic activity and strong structural selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1S, 3R, 5S)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-ethyl carboxylate 1 and (1R, 3R, 5R)-N-tert-butoxy Preparation of ethyl carbonyl-2-azabicyclo[3,1,0]hexane-3-carboxylate 2
[0052] in N 2 Under protection, add ethyl (R)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxylate (manufactured by Shanghai Aladdin Reagent Co., Ltd., CAS No.: 72925-16-7) (24.1g, 0.1mol) was dissolved in 200ml of dichloromethane (DCM), cooled to -15°C, diethylzinc (Et 2 Zn) (1.2eq, 0.12mol), stirred for 0.5h after the dropwise addition, added dropwise chloroiodomethane (CH 2 ClI) (1.1eq, 0.11mol) in dichloromethane (DCM) solution, maintain the temperature at -15 ° C and stir for 24h, after the reaction is completed, it is 13% ethylenediaminetetraacetic acid (EDTA) aqueous solution to quench Extinguish the reaction, heat up to 25°C, stir for 2 hours, let stand for 20 minutes, extract, combine the organic phases to obtain a yellow oil, add a 30% aqueous solution of diethylamine ...
Embodiment 2
[0061] Preparation of (1S, 3R, 5S)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-diphenylmethanol 3
[0062] In a 100mL two-necked flask, magnesium (3g, 120mmol) and a catalytic amount of iodine were added, under N 2 Slowly add the ether solution of bromobenzene (240mmol) dropwise under protection. When the reaction is initiated, keep the reaction temperature at 75°C, and the system will boil slightly. )-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-carboxylic acid ethyl ester 1 (14.02g, 55mmol) in ether solution (200mL), reflux, TLC confirmed that the raw material was completely After the reaction, add saturated NH 4 Quench the reaction with aqueous Cl solution, extract with diethyl ether (50mL×3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter with suction, remove the solvent by rotary evaporation under reduced pressure, column chromatography ethyl acetate / petroleum ether is 3 / 5, the white solid 3 was obtained, name...
Embodiment 3
[0067] Preparation of (1R, 3R, 5R)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-diphenylmethanol 3
[0068] Add magnesium (1.5g, 60mmol) and catalytic amount of iodine in a 100mL two-necked flask, 2 Slowly add the ether solution of bromobenzene (120mmol) dropwise under protection. When the reaction is initiated, keep the reaction temperature at 75°C, and the system will boil slightly. After the dropwise addition, reflux for 2h, and add the newly prepared Grignard reagent dropwise to (1R3R, 5R)- Ethyl N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-carboxylate 1 (7.01g, 25.75mmol) in ether solution (100mL), reflux, TLC confirmed the complete reaction of raw materials After adding saturated NH 4 Quench the reaction with aqueous Cl solution, extract with diethyl ether (50mL×3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter with suction, remove the solvent by rotary evaporation under reduced pressure, column chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com