Prolinol derivative induced chiral MOFs material with asymmetric catalysis
A derivative and asymmetric technology, applied in the field of chiral catalytic materials, can solve the problems of unobtained crystal structure and high-density catalytic sites, no enantioselectivity, and unexplainable mechanism, etc., so as to be easy to promote on a large scale Application, cheap raw materials, effect of low raw material prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 (synthesis of catalyst)
[0033] L-N-tert-butoxycarbonylprolinol (11.3g, 56mmol) 100mL pyridine solution was slowly added p-toluene chloride (13.1g, 68.8mmol) at 0°C and stirred overnight, diluted with 350mL of ethyl acetate and washed with 1N HCl (200mL×5), saturated NaHCO 3 (150mL×2) and brine (100mL×2) were extracted, the organic layer was dried over anhydrous sodium sulfate, and spin-dried to obtain a yellow oil. Take yellow oil (6.6g, 18.6mmol) and add imidazole sodium salt (2.51g, 27.9mmol) to 60mL of acetonitrile solution, heat to reflux for 1.5h, cool to room temperature, extract with chloroform, spin dry, and obtain light yellow solid L-N-tert Butoxycarbonyl-2-imidazole-1-pyrrolidine (BCIP). 1 H-NMR (300MHz, CDCl 3): d 1.27-1.28(1H, m), 1.48(9H, s), 1.60-1.75(2H, br), 1.89-1.94(1H, m), 3.14-3.37(2H, m), 3.99-4.08( 2H, m), 4.22-4.26 (1H, m), 6.87 (1H, s), 7.04 (1H, s), 7.44 (1H, s).
Embodiment 2
[0034] Embodiment 2 (synthesis of catalyst)
[0035] Isophthalic acid (99 g, 0.6 mol), 300 mL of oleum with a concentration of 50%, and paraformaldehyde (9.3 g, 0.3 mol) were successively added into a 1000 ml round bottom flask. The mixture was stirred at 118 °C for 6 h, then the heating was stopped. After cooling to room temperature, the reaction mixture was carefully poured into a glass container filled with a large amount of ice cubes, stirred with a glass rod for about 20 min, left to stand for a period of time and then suction filtered, and the light yellow precipitate obtained by suction filtration was vacuum dried. The yellow solid obtained from the above reaction was dissolved in 300 mL of saturated methanolic HCl solution, refluxed at 95° C. for 1 h, cooled to room temperature, and the reaction mixture was suction-filtered to obtain a yellow crude product. The crude product was dissolved in 300ml of chloroform, the insoluble matter was filtered off, and the filtrate ...
Embodiment 3
[0037] Embodiment 3 (synthesis of catalyst)
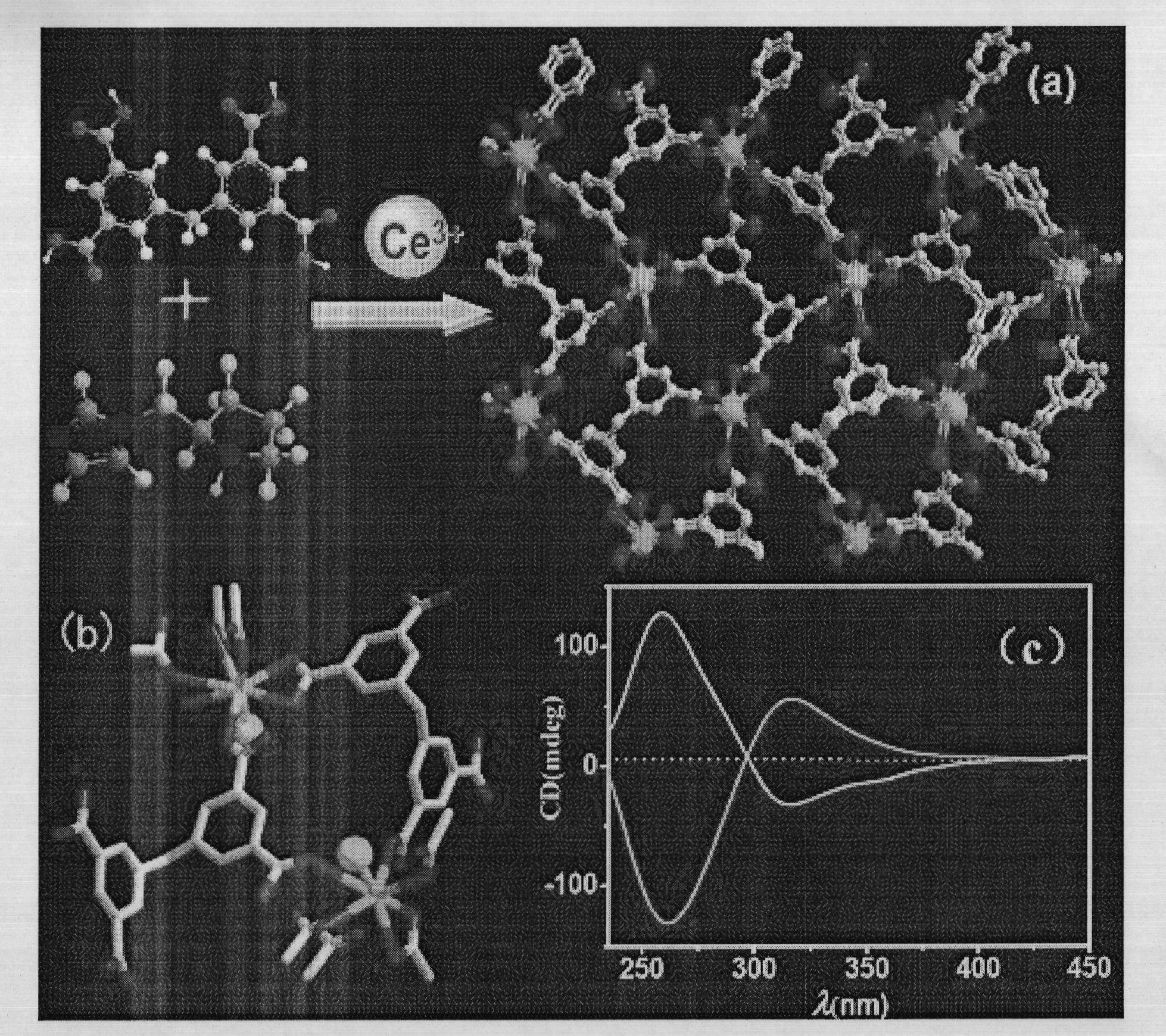
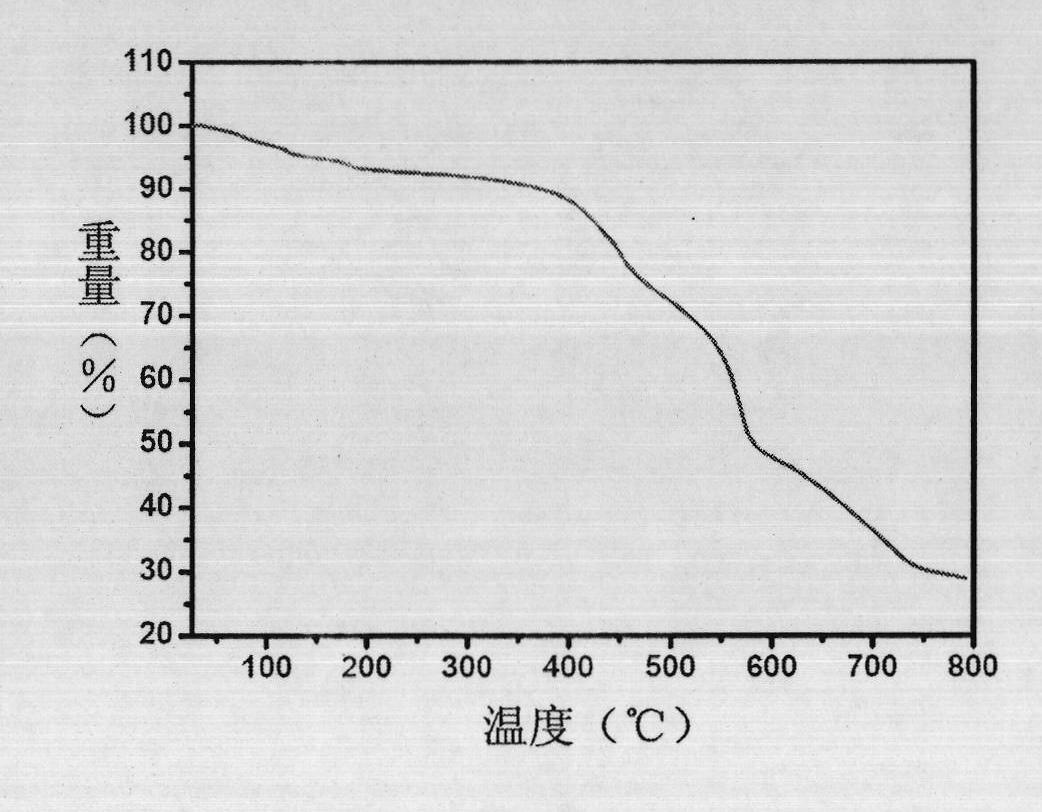
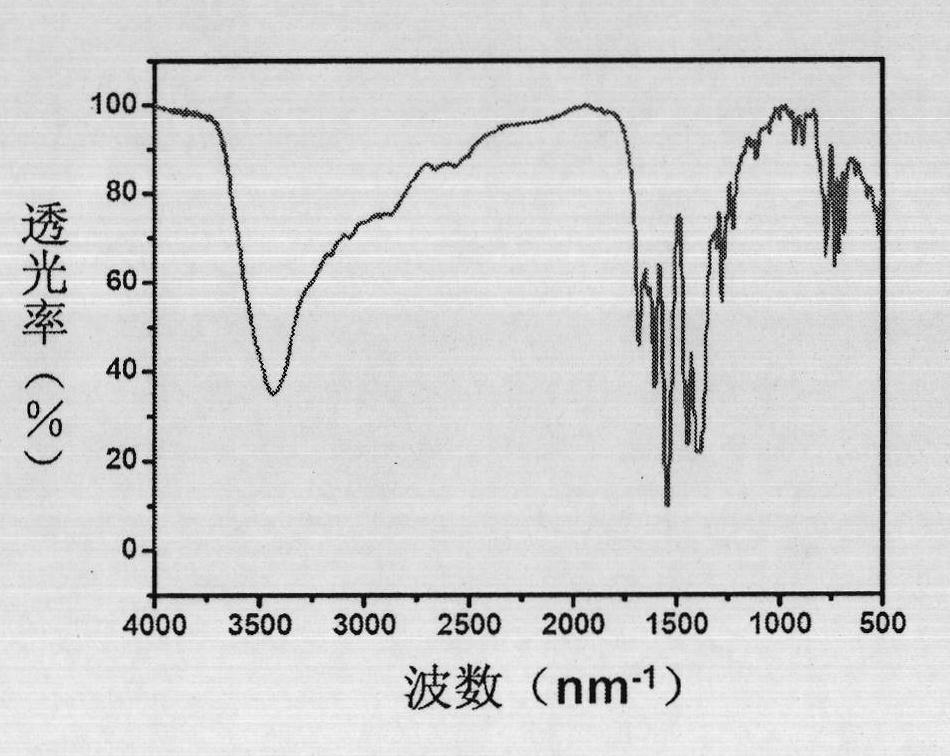
[0038] 5,5'-methylene diisophthalic acid (H 4 MDIP) (0.086g, 0.25mmol), cerium nitrate hexahydrate (0.0217g, 05mmol), L-N-tert-butoxycarbonyl-2-imidazole-1-pyrrolidine (0.125g, 0.5mmol) were dissolved in water, and washed with triphenylamine Adjust the pH value of the solution to about 6, stir it evenly, place it in an oven, burn it at 100°C for 3 days, turn off the oven, cool to room temperature, colorless massive crystals are produced, filter and dry, the yield is 60%. Anal calc. for C 17 h 11 CeO 9 ·H 2 O: C 39.46, H 2.53%; Found: C 38.53, H 2.91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com