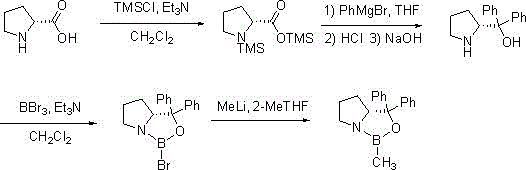Preparation method of chiral CBS catalyst
A catalyst and chiral technology, applied in the field of preparation of chiral CBS catalysts, can solve the problems of cumbersome operation, long reaction steps, increased cost, etc., and achieve the effect of shortening the reaction steps and simplifying the operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
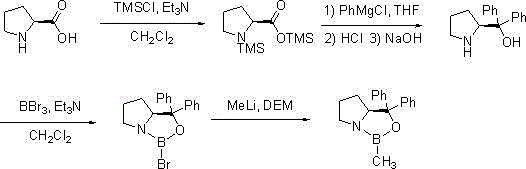
[0023] Synthesis of S-MeCBS:
[0024]
[0025] The first step: In the reaction flask, add L-proline (115g, 1mol), triethylamine (253g, 2.5mol) and dichloromethane (1600mL) in sequence, cool to -5°C to 5°C, drop Chlorotrimethylsilane (239g, 2.2mol), after the reaction was completed, the reaction solution was evaporated to dryness, after adding 650mL of toluene, the triethylamine hydrochloride produced during the reaction was filtered off, and the filtrate was directly used in the next step after evaporation;
[0026] The second step: add the product of the first step above into 850mL tetrahydrofuran, cool to -10°C to 0°C, slowly add 2M phenylmagnesium chloride (1.1L, 2.2mol) dropwise, after the reaction is completed, add 15% hydrochloric acid aqueous solution to adjust the pH = 1-2, filter the generated off-white solid, add 15% sodium hydroxide to adjust PH=10-12, extract four times with dichloromethane (1500mL), combine the organic layers, dry over anhydrous magnesium sul...
Embodiment 2
[0030] Synthesis of R-MeCBS:
[0031]
[0032] The first step: In the reaction flask, add D-proline (115g, 1mol), triethylamine (253g, 2.5mol) and dichloromethane (1600mL) successively, cool to -10°C to -5°C, drop Add trimethylchlorosilane (239g, 2.2mol), after the reaction is completed, evaporate the reaction solution to dryness, add 650mL of toluene, filter out the triethylamine hydrochloride produced during the reaction, and directly use the filtrate in the next step after evaporation ;
[0033] The second step: add the product of the first step above into 850mL tetrahydrofuran, cool to -10°C to 0°C, slowly add 1M phenylmagnesium bromide (2.2L, 2.2mol) dropwise, after the reaction is completed, add 15% hydrochloric acid aqueous solution Adjust the pH to 1-2, filter the generated off-white solid, add 15% sodium hydroxide to adjust the pH to 10-12, extract four times with dichloromethane (1700mL), combine the organic layers, dry over anhydrous magnesium sulfate, and dis...
Embodiment 3
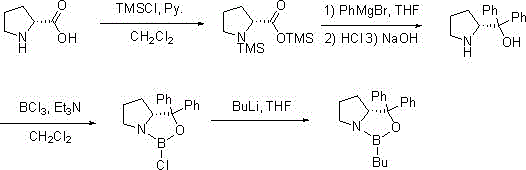
[0037] Synthesis of R-BuCBS:
[0038]
[0039] Step 1: In the reaction flask, add D-proline (115g, 1mol), pyridine (172g, 2.2mol) and dichloromethane (1600mL) in sequence, cool to -5°C to 5°C, add trimethyl Chlorochlorosilane (239g, 2.2mol), after the completion of the reaction, evaporate the reaction solution to dryness, add 700mL of toluene, filter out the pyridine hydrochloride produced in the reaction process, and directly use the filtrate in the next step after evaporation;
[0040]The second step: Add the above-mentioned first step product into 850mL tetrahydrofuran, cool to -10°C to 0°C, slowly add 1M phenylmagnesium bromide (2.2L, 2.2mol) dropwise, after the reaction is completed, add 10% hydrochloric acid aqueous solution Adjust the pH to 1-2, filter the resulting off-white solid, add 10% sodium hydroxide to adjust the pH to 10-12, extract four times with dichloromethane (1800mL), combine the organic layers, dry over anhydrous magnesium sulfate, and distill the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com