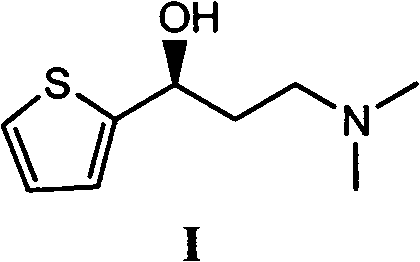
Asymmetric synthesis method of duloxetine intermediate-(S)-N, N-dimethyl-3-hydroxy-3-(2-thienyl)-1-propylamine
A technology of thienyl and dimethyl, applied in the field of asymmetric synthesis of chiral alcohols, can solve the problems of oxygen contraindication and limited application, and achieve the effect of avoiding loss and saving dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1.1 (S)-N, the synthesis of N-dimethyl-3-hydroxyl-3-(2-thienyl) propylamine (I)
[0037]
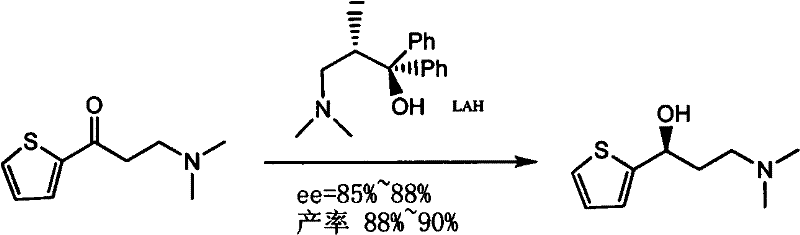
[0038] In a 50ml three-necked flask, add 0.74g (4.0mmmol) 3-dimethylamino-1-(2-thienyl)-1-propanone and 0.16g (0.6mmol) α, α-diphenylprolinol successively And 10ml absolute ethanol, stir. Add 0.16 g of sodium borohydride to the reaction solution under ice-bath condition, remove the ice-bath, warm up to room temperature to continue the reaction, follow the reaction by TLC spotting until the reaction is complete. Stirring was stopped, rotary evaporation under reduced pressure, dissolved in water, extracted with ethyl acetate (20ml×3), the organic layer was collected and dried over anhydrous MgSO4. Suction filtration, rotary evaporation under reduced pressure, obtain 0.465g product, yield 62.8%, [α] D 22 =-7.46 (c, 1.0, MeOH) (literature value ] :[α] D 2.5 = -7.6. (c, 1.0, MeOH)), ee = 98%.
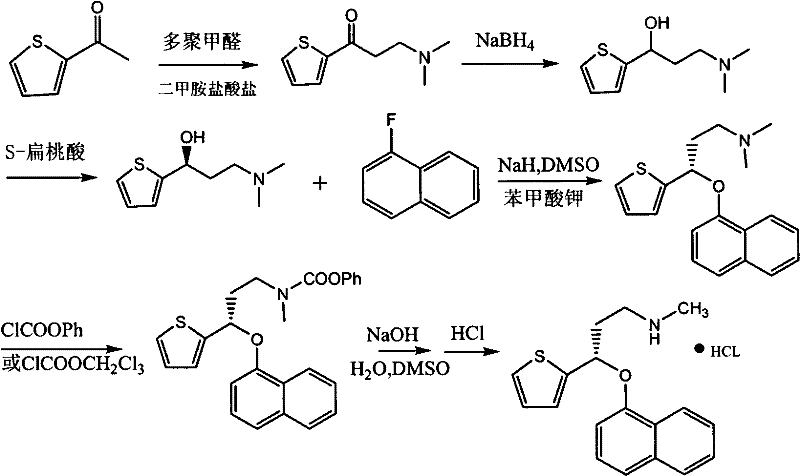
[0039] 1.2 Preparation of (S)-N, N-dimethyl-3-(1-naphthyloxy)-3-(2-thienyl)-1-prop...
Embodiment 2
[0052] Embodiment 2 (S)-N, the synthesis of N-dimethyl-3-hydroxyl-3-(2-thienyl) propylamine (I)
[0053]
[0054] Add 7.4 grams of 3-dimethylamino-1-thienyl)-1-acetone (40mmol), 1.6 grams of α, α-diphenylprolinol (6mmol) and 50ml of absolute ethanol in the there-necked flask, and stir in an ice bath 1.6 g of sodium borohydride (40 mmol) were added slowly in portions. Then remove the ice bath and stir at room temperature until the reaction is complete (about 2h). Spin off ethanol under reduced pressure, add 70ml of water to dissolve the residue, extract with ethyl acetate (20ml×3), dry the organic phase with anhydrous magnesium sulfate, filter with suction and spin dry to obtain a white solid. Recrystallization was carried out with 25 ml of petroleum ether to obtain 7 g of 3-dimethylamino-1-thienyl)-1-propanol, with a yield of 94.1%. [α] D 25 = -7.1 (c, 1.0, MeOH).
Embodiment 3
[0055] Embodiment 3 (S)-N, the synthesis of N-dimethyl-3-hydroxyl-3-(2-thienyl) propylamine (I)
[0056] Divided by (R)-(+)-α, α-diarylprolinol silicon ether (0.6mmol) to replace (R)-(+)-α, α-diarylprolinol (0.6mmol ), the others are the same as in Example 1, the yield of (S)-N,N-dimethyl-3-hydroxyl-3-(2-thienyl)propylamine (I) is 77.8%, ee=99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com