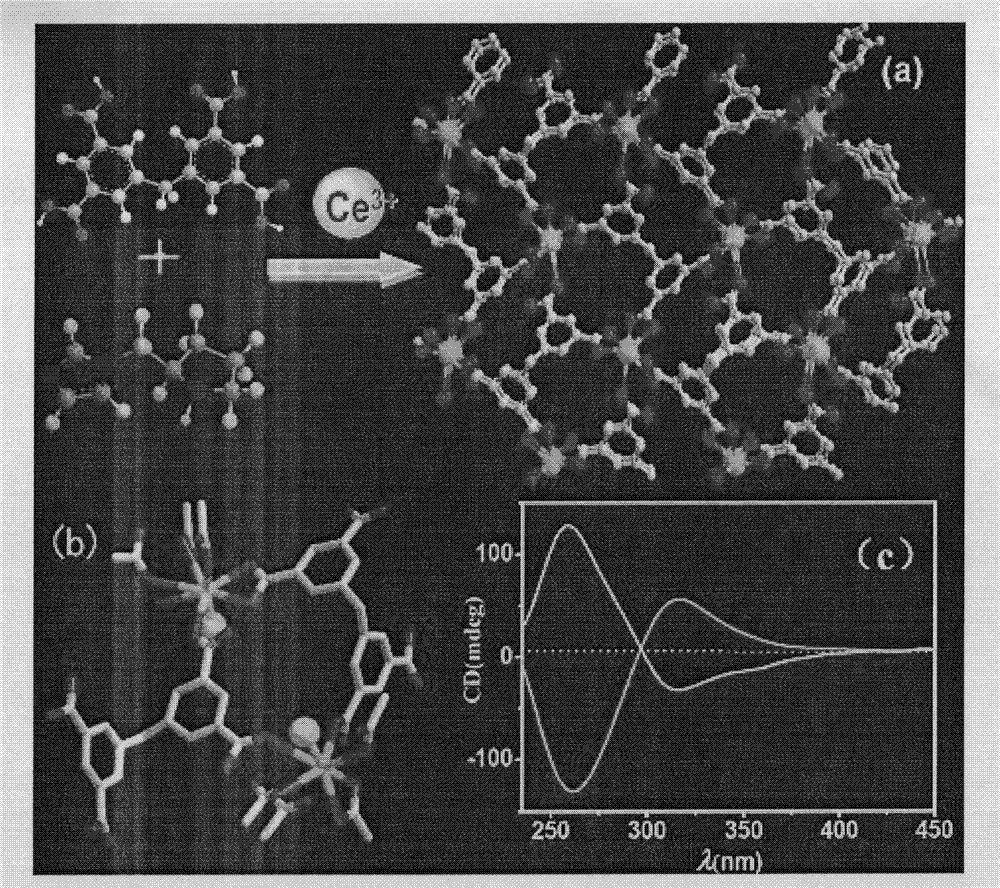
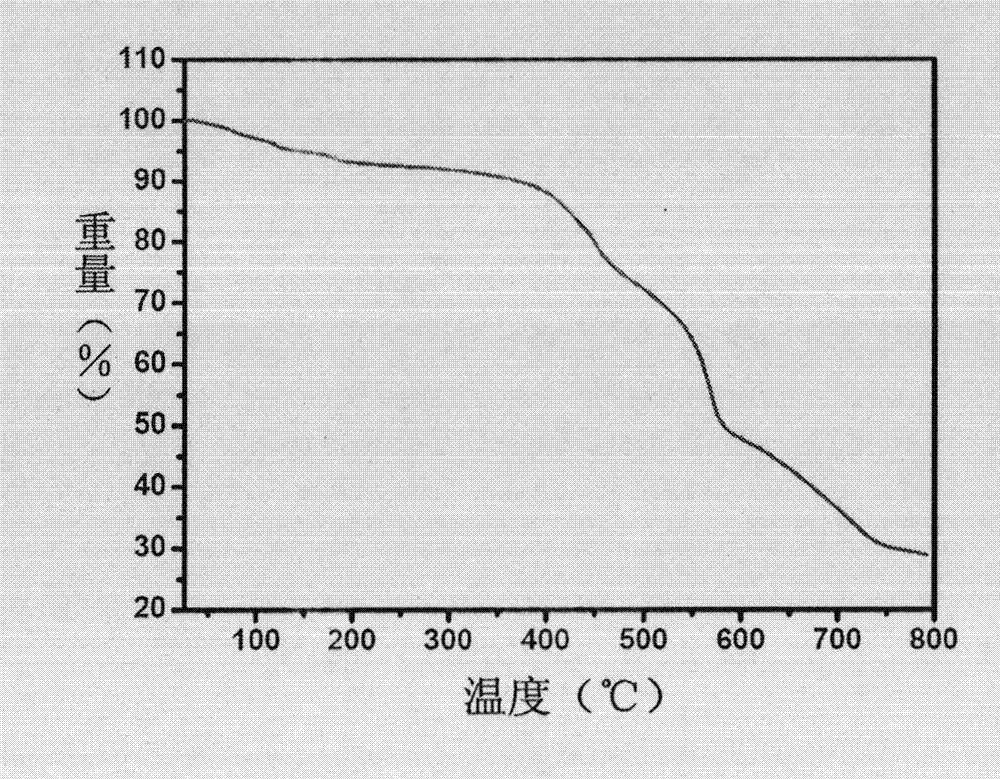
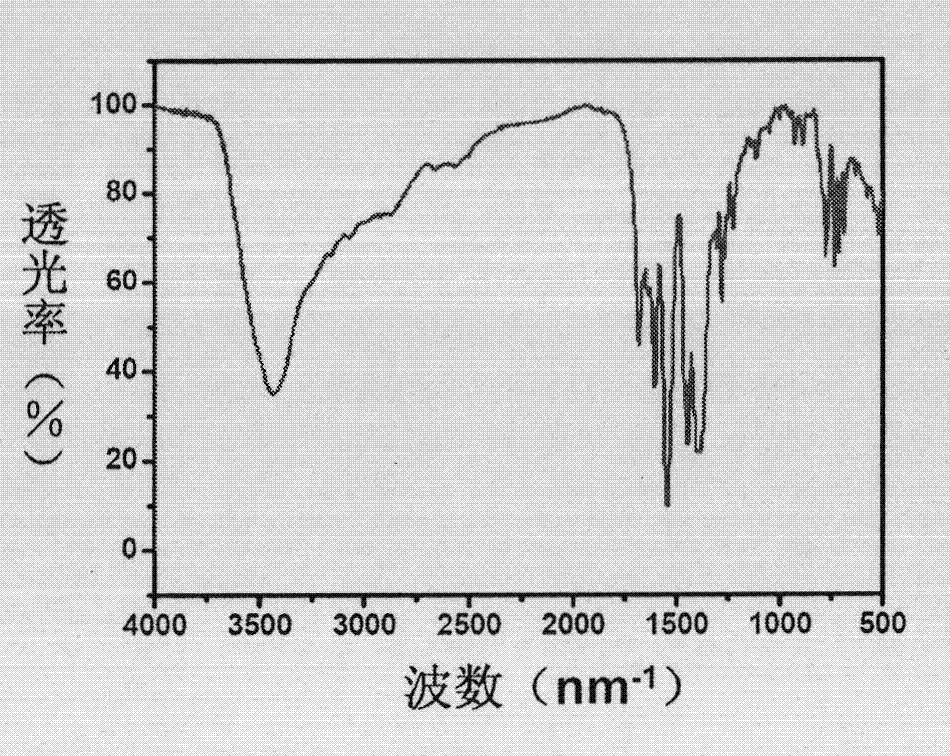
Prolinol derivative induced chiral MOFs material with asymmetric catalysis
A derivative and asymmetric technology, which is applied in the field of chiral catalytic materials, can solve the problems of unobtained crystal structure and high-density catalytic sites, no enantioselectivity, low catalytic efficiency, etc., and is easy to promote on a large scale Application, cheap raw materials, effect of low raw material prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 (Synthesis of catalyst)
[0033] LN-tert-Butoxycarbonylprolinol (11.3g, 56mmol) 100mL pyridine solution was slowly added to p-toluene yellow acyl chloride (13.1g, 68.8mmol) at 0°C and stirred overnight, diluted with 350mL of ethyl acetate and respectively used 1N HCl (200mL×5), saturated NaHCO 3 (150 mL×2) and brine (100 mL×2) were extracted, the organic layer was dried over anhydrous sodium sulfate, and spin-dried to obtain a yellow oil. Take the yellow oily substance (6.6g, 18.6mmol) and add imidazole sodium salt (2.51g, 27.9mmol) to 60mL acetonitrile solution and heat to reflux for 1.5h, cool to room temperature, extract with chloroform, spin dry to obtain a light yellow solid LN-tert Butoxycarbonyl-2-imidazole-1-pyrrolidine (BCIP). 1 H-NMR(300MHz, CDCl 3 ): d 1.27-1.28 (1H, m), 1.48 (9H, s), 1.60-1.75 (2H, br), 1.89-1.94 (1H, m), 3.14-3.37 (2H, m), 3.99-4.08 ( 2H, m), 4.22-4.26 (1H, m), 6.87 (1H, s), 7.04 (1H, s), 7.44 (1H, s).
Embodiment 2
[0034] Example 2 (Synthesis of catalyst)
[0035] In a 1000ml round bottom flask, Isophthalic acid (99g, 0.6mol), 300mL of oleum with a concentration of 50%, and paraformaldehyde (9.3g, 0.3mol) were sequentially added. The mixture was stirred and reacted at 118°C for 6 hours, and then the heating was stopped. After being cooled to room temperature, the reaction mixture was carefully poured into a glass container containing a large amount of ice cubes, stirred with a glass rod for about 20 minutes, and then allowed to stand for a period of time and then filtered with suction, and the light yellow precipitate obtained by the suction filtered was vacuum dried. The yellow solid obtained from the above reaction was dissolved in 300 mL of saturated methanolic HCl solution, and refluxed at 95° C. for 1 h. After cooling to room temperature, the reaction mixture was suction filtered to obtain a yellow crude product. The crude product was dissolved in 300 ml of chloroform, the insoluble m...
Embodiment 3
[0037] Example 3 (Synthesis of catalyst)
[0038] 5,5’-Methylene diisophthalic acid (H 4 MDIP) (0.086g, 0.25mmol), cerium nitrate hexahydrate (0.0217g, 05mmol), LN-tert-butoxycarbonyl-2-imidazole-1-pyrrolidine (0.125g, 0.5mmol) dissolved in water, and triphenylamine Adjust the pH value of the solution to about 6, after stirring evenly, place it in an oven at 100°C for 3 days, close the oven, cool to room temperature, colorless lumpy crystals are produced, filtered and dried, and the yield is 60%. Anal calc.for C 17 H 11 CeO 9 ·H 2 O: C 39.46, H 2.53%; Found: C 38.53, H 2.91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com