L-prolinol synthetic method
A synthesis method and a technology for prolinol, applied in directions such as organic chemistry, can solve the problems of high price, high cost, undesired production, etc., and achieve the effects of safe reaction temperature, low safety factor, and strong competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
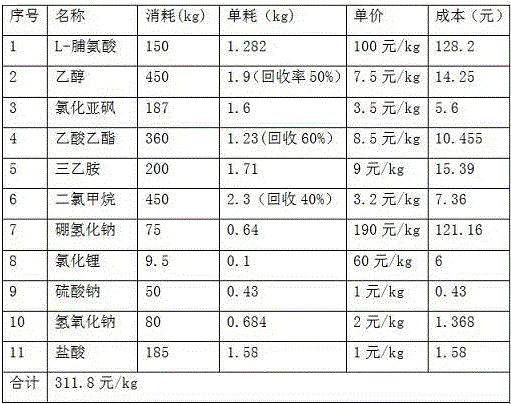
[0017] 1) Esterification:
[0018] Add 450kg of ethanol to the 1000L reaction kettle, start stirring, turn on the brine to cool down to below 10 degrees, add 187kg of thionyl chloride dropwise, control the temperature at 10-15 degrees, add L-proline 150kg;
[0019] After the addition of L-proline, the temperature was raised to 40°C, and the reaction was incubated for 10 hours to see the end of the reaction by TLC. After the reaction, concentrate under reduced pressure at 50 degrees to recover ethanol to the certificate, add 380kg of ethyl acetate, adjust the pH to 7-8 with triethylamine, and filter. Mother liquor reclaims ethyl acetate, obtains oily matter L-proline ethyl ester 190kg;
[0020] 2) Restore:
[0021] Add 800kg of methanol and 190kg of L-proline ethyl ester into a 2000L reaction kettle and start stirring. Add 9.5kg of lithium chloride at 5-10 degrees to cool down, and add 75kg of sodium borohydride in portions. It takes 2 hours to complete the addition and heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com