Use of aryloxy-functionalized prolinol chiral ligand as catalyst
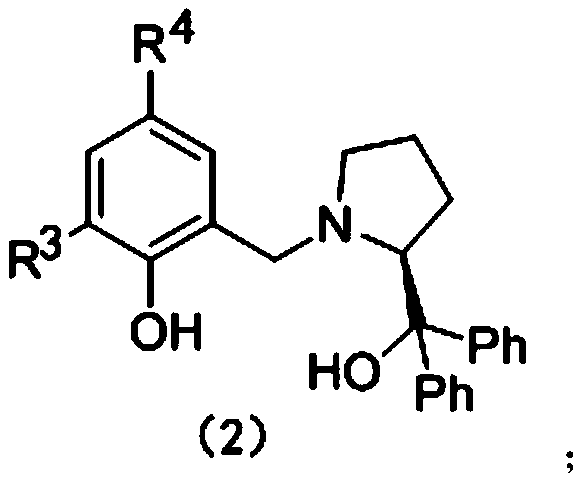
A technology for synthesizing prolinol and chiral ligands, which is applied in the application field of aryloxy-functionalized prolinol chiral ligands as catalysts, can solve problems such as rising enantioselectivity of products, and achieve good enantioselectivity , Universal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
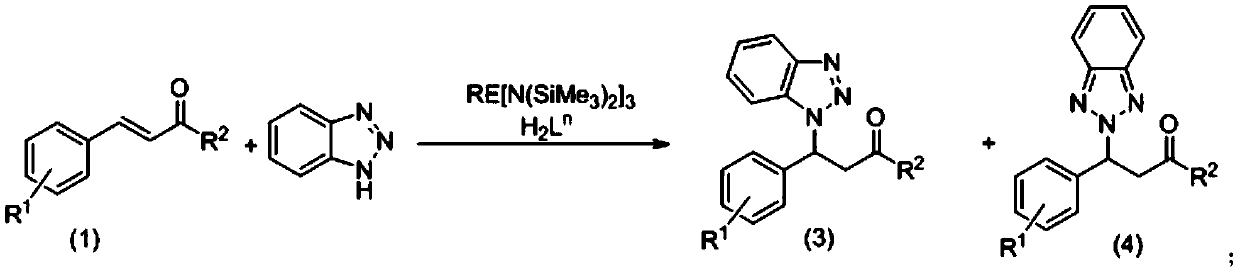
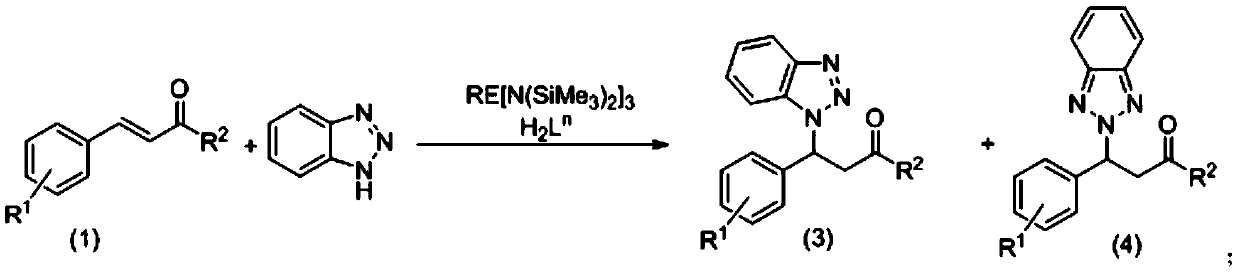
[0053] Example 1: Aryloxy functionalized chiral prolinol ligands combined with rare earth metal amides catalyze the asymmetric reaction of chalcones and benzotriazoles:
[0054] Under argon protection, add 0.0179g (0.03×10 -3 mol) Sc[N(SiMe 3 ) 2 ] 3 , and then adding the aryloxy functionalized chiral prolinol ligand H 2 L 4 0.0249g (0.03×10 -3 mol), add 1 mL of chloroform solvent to dissolve it, stir for 0.5 h, add chalcone 0.0624 (0.3×10 -3 mol), continue to stir the reaction for 30min, then add 0.0429g (0.36×10 -3 mol) benzotriazole, sealed the bottle and stirred for 24 hours, terminated the reaction with water, added 3mL ethyl acetate for extraction, took the upper organic phase, added silica gel and spin-dried, column chromatography (EA:PE=1:10), obtained hand The sum of the reaction conversions of the obtained products 3(a) and 4(a) is 87%. The yield ratio of 3(a) and 4(a) was close to 7:2, and the ee value of 3(a) reached 75%.
[0055] The structure of product...
Embodiment 2
[0062] Example 2: Aryloxy-functionalized chiral prolinol ligands and rare earth metal amides jointly catalyze the asymmetric reaction of 4-chlorochalcone and benzotriazole:
[0063] Under argon protection, add 0.0179g (0.03×10 -3 mol) Sc[N(SiMe 3 ) 2 ] 3 , and then adding the aryloxy functionalized chiral prolinol ligand H 2 L 4 0.0249g (0.06×10 -3 mol), add 1 mL of chloroform solvent to dissolve it, stir the reaction for 0.5 h, add 0.0726 g of 4-chlorochalcone (0.3×10 -3 mol), continue to stir the reaction for 30min, then add 0.0429g (0.36×10 -3 mol) of benzotriazole, sealed the bottle and stirred for 24 hours, terminated the reaction with water, added 3 mL of ethyl acetate for extraction, took the upper organic phase, added silica gel and spin-dried, column chromatography (EA:PE=1:10), obtained The chiral addition product has a conversion rate of 84%, the yield ratio of product 3(b) and 4(b) is close to 64:20, and the ee value of product 3(b) reaches 61%.
[0064] Th...
Embodiment 3
[0070]Example 3: Aryloxy-functionalized chiral prolinol ligands and rare earth metal amides jointly catalyze the asymmetric reaction of 4-fluorochalcone and benzotriazole:
[0071] Under argon protection, add 0.0179g (0.03×10 -3 mol) Sc[N(SiMe 3 ) 2 ] 3 , and then adding the aryloxy functionalized chiral prolinol ligand H 2 L 4 0.0249g (0.06×10 -3 mol), add 1 mL of chloroform solvent to dissolve it, stir the reaction for 0.5 h, add 0.0678 g of 4-fluorochalcone (0.3×10 -3 mol), continue to stir the reaction for 30min, then add 0.0429g (0.36×10 -3 mol) of benzotriazole was sealed and stirred for 24 hours, terminated the reaction with water, added 3mL of ethyl acetate for extraction, took the upper organic phase, added silica gel and spin-dried, column chromatography (EA:PE=1:10), obtained hand The reaction conversion rate is 95%, the yield ratio of product 3(c) and 4(c) is close to 67:28, and the ee value of product 3(c) reaches 71%.
[0072] The structure of product 3(c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com