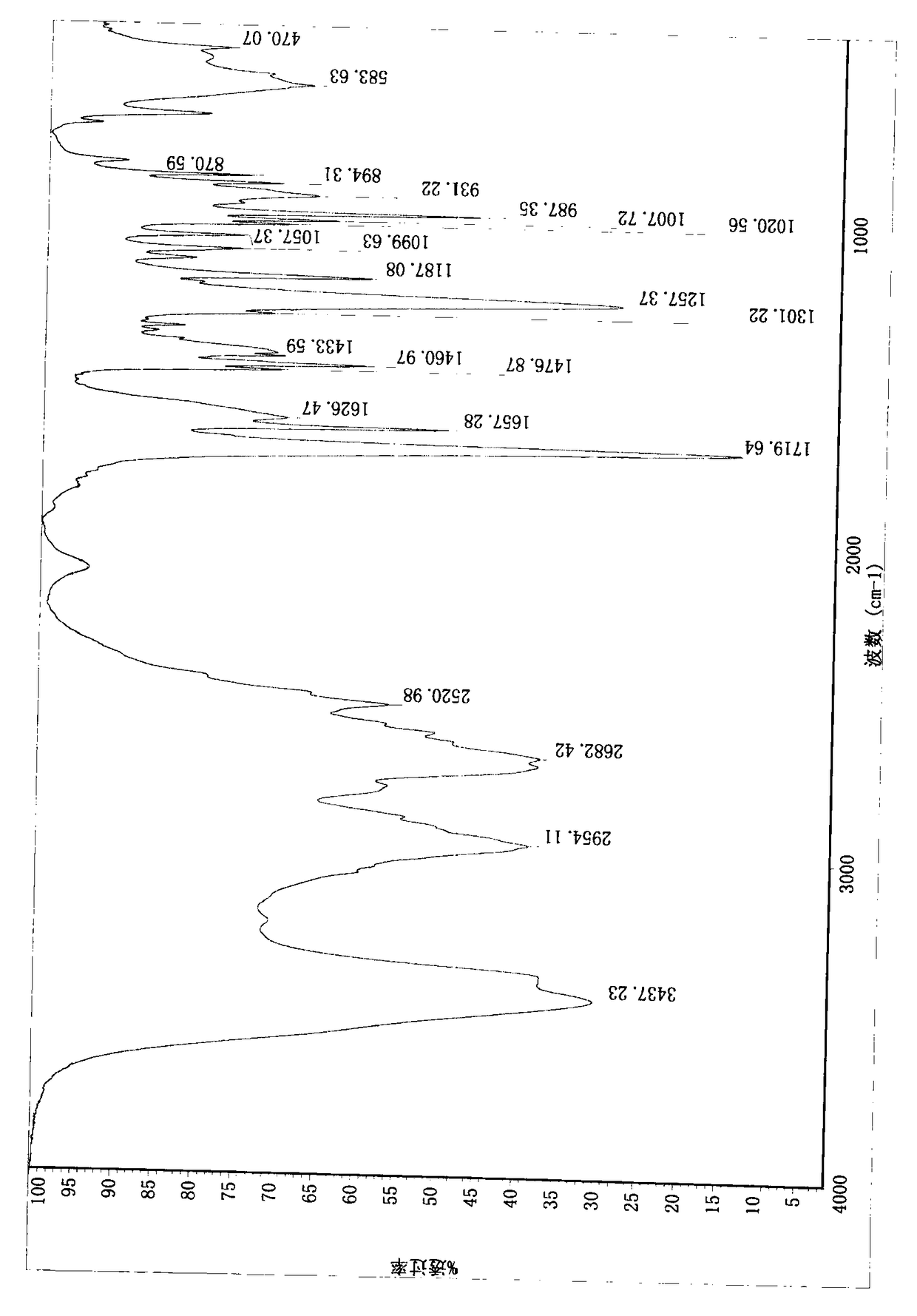
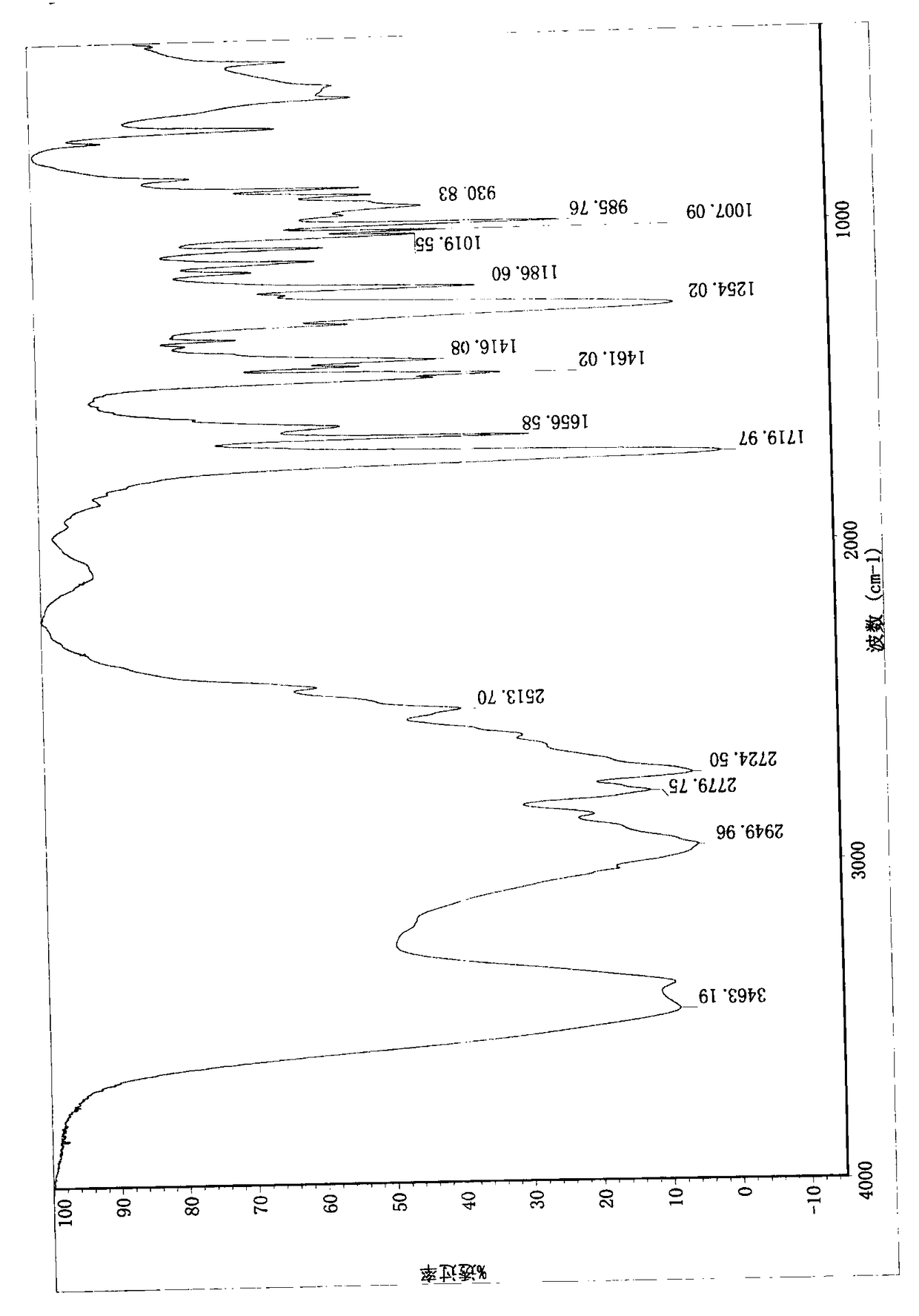
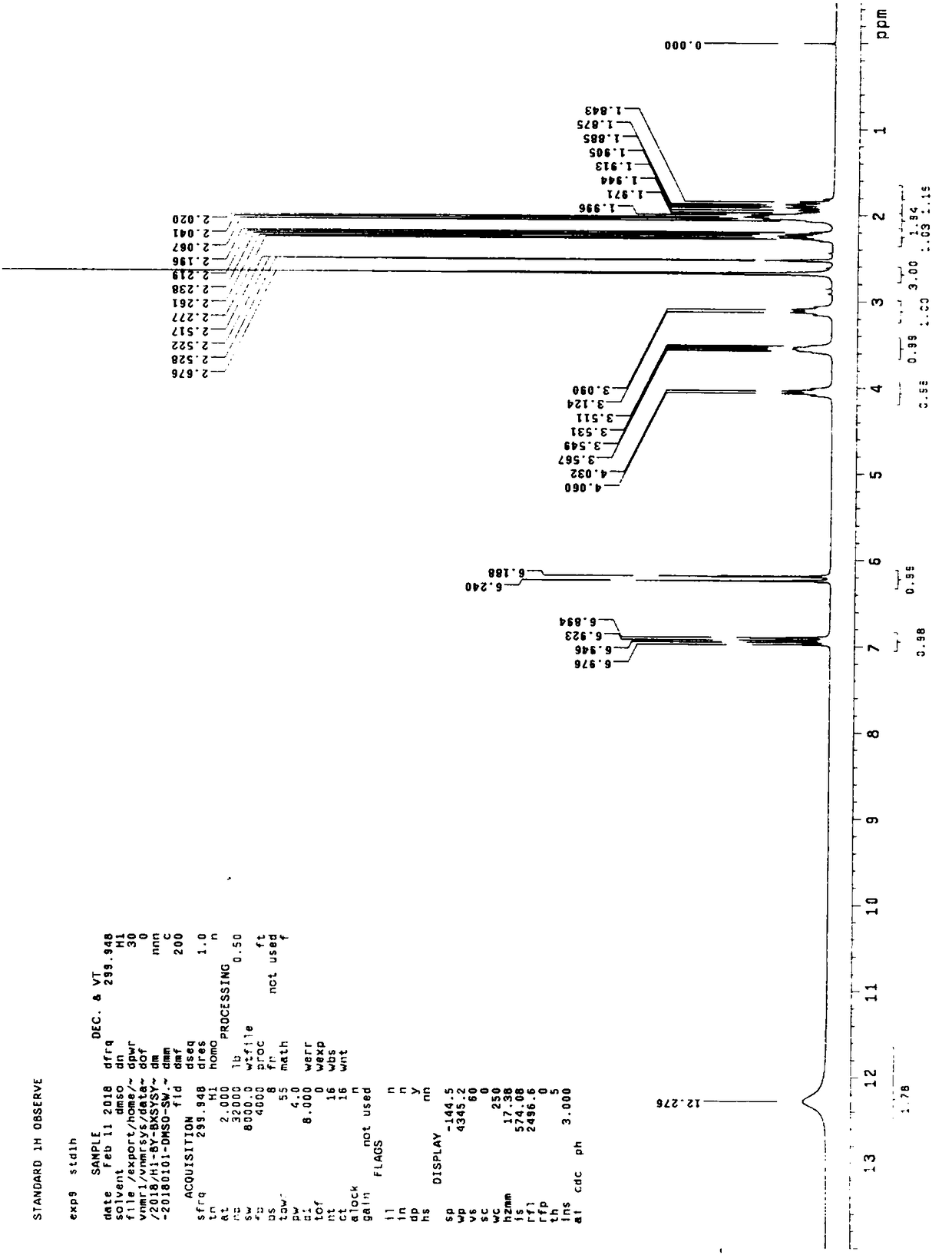
Compound (E)-3-(1-methylpyrrolidine-2-yl)-acrylic hydrochloride and synthetic method
A technology of acrylic acid hydrochloride and methylpyrrolidine, which is applied in the field of compound-3-acrylic acid hydrochloride and its synthesis, which can solve the problems of low yield, easy to be oxidized, and difficult to react in the next step, so as to simplify the reaction process , stable structure, and the effect of improving the total yield of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Synthesis of (S, E)-3-(1-methylpyrrolidin-2-yl)-acrylic acid hydrochloride
[0055] 1) Synthesis of BOC-L-prolinaldehyde
[0056] Weigh 100g of BOC-L-prolinol into a 2L four-necked reaction flask, add 1.5L of dichloromethane, stir and dissolve at room temperature, add 15.4g of sodium bromide, 2.3g of TEMPO, cool down below -10°C, and start dripping Add sodium hypochlorite solution adjusted to pH=8~9 in advance, and keep the temperature at -5~-10°C. TLC tracking (developer ethyl acetate:petroleum ether=1:1), stop adding sodium hypochlorite solution dropwise (about 500ml) after the reaction of the raw materials is complete, static layering, leave the organic layer, and use 1% sulfuric acid solution 800ml, Wash once each with 800 ml of 5% sodium thiosulfate solution and 800 ml of saturated saline, dry the organic layer over anhydrous magnesium sulfate, filter, and evaporate to dryness to obtain 94 g of BOC-L-prolinaldehyde.
[0057] 2) Synthesis of S-pyrrolidi...
Embodiment 2
[0066] Example 2: (S,E)-N-﹛4-[3-chloro-4-(pyridin-2-ylmethoxy)-anilino]-3-cyano-7-ethoxy-quinoline Synthesis of -6-yl﹜-3-(1-methyl-pyrrolidin-2-yl)-acrylamide (compound 1)
[0067]
[0068] Add 7.7 g of (S,E)-3-(1-methylpyrrolidin-2-yl)-acrylic acid hydrochloride obtained in Example 1 into a reaction flask with 60 ml of tetrahydrofuran, add 1 ml of DMF, and stir Add 7.6g of oxalyl chloride dropwise, control the temperature at 20-25°C, keep warm and stir for 2-3 hours; cool the reaction solution to -5-0°C with a cold pump, add 4-[4-[(2-pyridyl)formazol dropwise Oxy]-3-chloroanilino]-6-amino-3-cyano-7-ethoxyquinoline in tetrahydrofuran (4-[4-[(2-pyridyl)methoxy]-3- Chloroanilino]-6-amino-3-cyano-7-ethoxyquinoline 16.3g, tetrahydrofuran 70ml), keep the temperature below 0°C, keep it warm for 1-2h after the dropwise addition, add 24g of triethylamine dropwise, Stir at 0-5°C for 4-5 hours, add 2ml of methanol at this temperature, concentrate the reaction solution to dryness af...
Embodiment 3
[0069] Example 3: Synthesis of (R, E)-3-(1-methylpyrrolidin-2-yl)-acrylic acid hydrochloride
[0070] 1) Synthesis of BOC-D-prolinaldehyde
[0071] Weigh 100g of BOC-D-prolinol and place it in a 2L four-necked reaction flask, add 1.5L of dichloromethane, stir and dissolve at room temperature, add 15.5g of sodium bromide and 2.5g of TEMPO, lower the temperature below -10°C, and start dripping Add sodium hypochlorite solution adjusted to pH=8~9 in advance, and keep the temperature at -5~-10°C. TLC tracking (developing agent ethyl acetate:petroleum ether=1:1), after the reaction of the raw materials is complete, stop adding sodium hypochlorite solution (about 500ml dropwise), static layering, keep the organic layer, and use 1% sulfuric acid solution 800ml respectively , 800ml of 5% sodium thiosulfate solution and 800ml of saturated saline were washed once respectively, and the organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com