Synthesis method of cis 3-phenyl substituted s-proline derivative
A technology of phenyl substitution and synthesis method is applied in the synthesis field of 3-phenyl substituted proline derivatives, can solve the problems of increasing reaction steps, low reaction conversion rate, reducing reaction yield and the like, and achieves mild reaction conditions, Good stereoselectivity and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
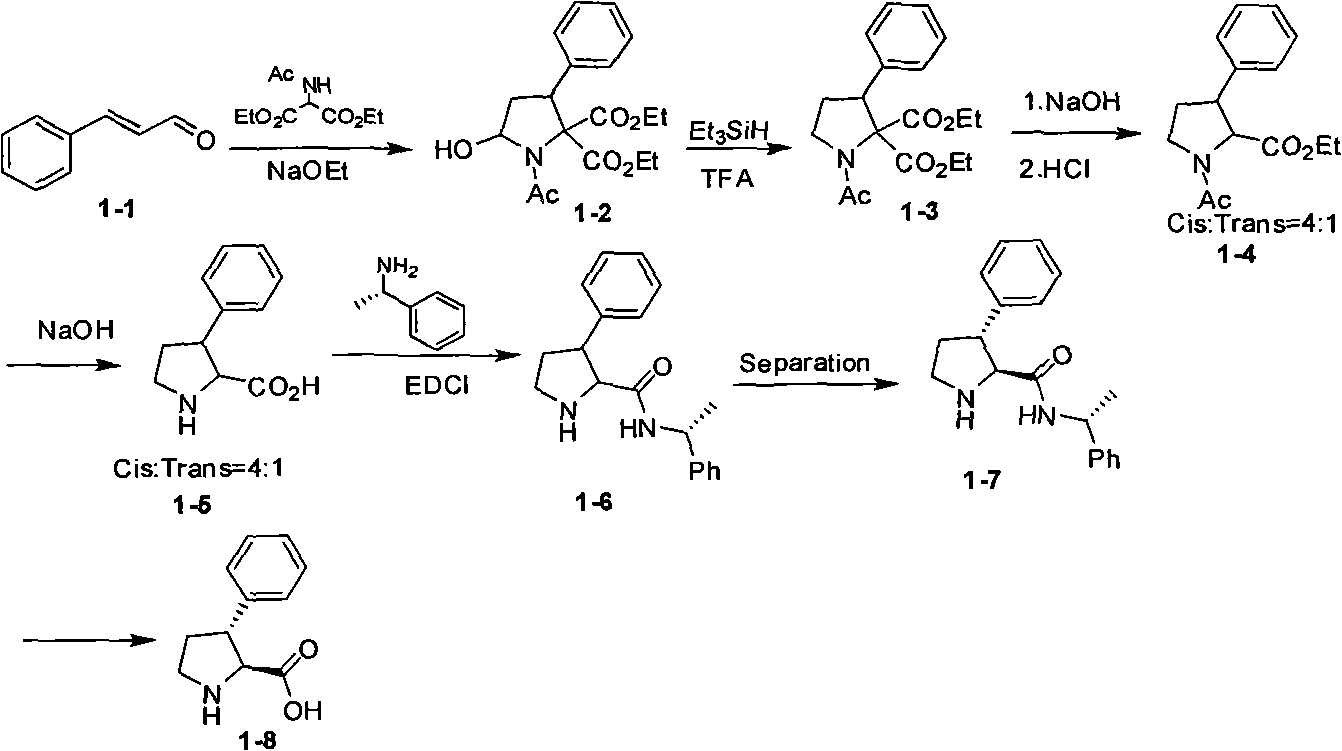
Embodiment 1
[0038] Example 1 Asymmetric Michael addition reaction
[0039]
[0040]a) Under nitrogen protection, cinnamaldehyde (132g, 1mol), (S)-diphenylprolinol trimethylsilyl ether (48g, 0.15mol), benzoic acid (36.6g, 0.3mol), nitroethanol (273g, 3mol) was added to methanol (5L), stirred overnight at room temperature and then added NaHCO 3 (420 g, 5 mol). Stirring was continued overnight at room temperature. Suction filtration, the solid was washed with methanol, methanol was distilled off under reduced pressure, the residue was added with water (1 L) and extracted with ethyl acetate, the organic phase was washed with dilute hydrochloric acid, washed with water, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain crude product. The crude product was recrystallized with 5 times of methanol to obtain 106 g of white solid, the yield was 47%, and the excess of optical isomer was more than 95%. Proton NMR data (CDCl3): 2.03 (1H, dt), 2.18...
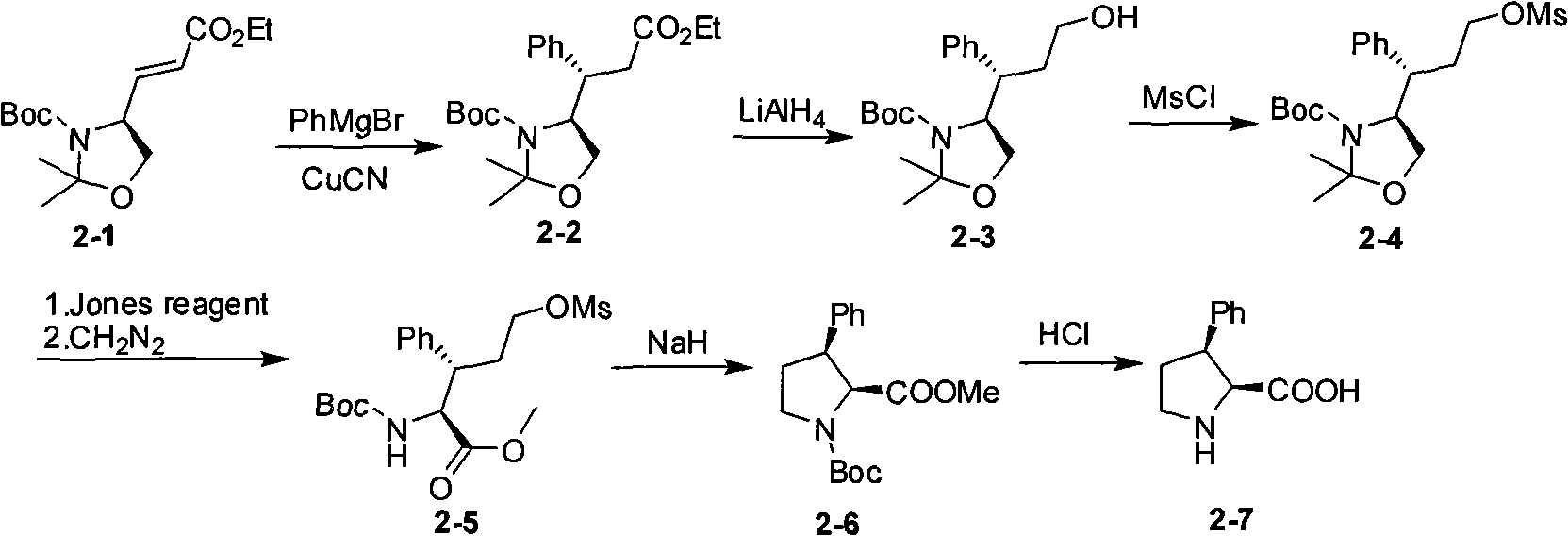
Embodiment 2
[0043] Example 2 Reduction and Boc Protection Reaction
[0044]
[0045] a) (4S, 5S)-5-nitro-4-phenyltetrahydro-2H-pyran-2-ol (22.3g, 0.1mol), palladium carbon catalyst (1g) and methanol (250m) were thrown into In the autoclave, the reaction was carried out under 300 psi hydrogen pressure for 16 hours, and TLC showed that the reaction was complete. After filtration, the filtrate was concentrated under reduced pressure to obtain an oil. The oil was dissolved in ethyl acetate (350ml) and BOC 2 O (22.8g, 0.1mol), N,N-dimethyl-4-pyridine (0.6g, 0.005mol), stirred overnight at room temperature, TLC showed that the reaction was complete. The solvent was removed under reduced pressure, and the residue was recrystallized from petroleum ether to obtain 16 g of a white solid with a yield of 58%. Proton NMR spectrum (CDCl3) 1.47 (9H, s), 2.24 (1H, quint, J=6.0Hz), 2.30, 2.50 (1H, m), 3.28-3.43 (1H, m), 3.46, 3.61 (2H, m), 3.60, 3.68 (1H, m), 4.28 (1H, bs), 7.21, 7.33 (3H, m), 7.32...
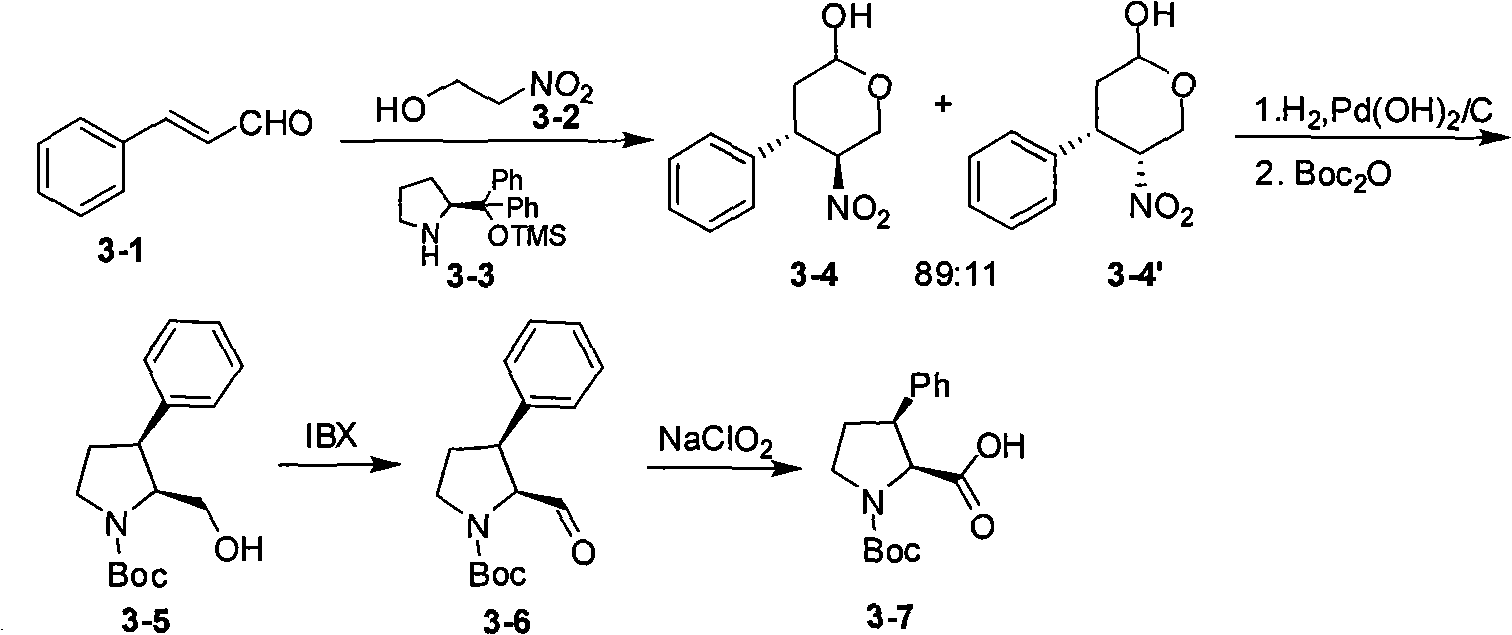
Embodiment 3
[0048] Example 3 Oxidation reaction
[0049]
[0050] a) input (2S, 3S)-tert-butyl-2-(hydroxymethyl)-3-phenylpyrroline-1-carboxylic acid (27.7g, 0.1mol), ethyl acetate (150ml), potassium bromide (2.4g, 0.02mol), tetramethylpiperidinium oxide (0.8g, 5mmol), under the cooling of ice-water bath, begin to drip sodium hypochlorite aqueous solution (300ml, effective rate 5%, 0.2mol), continue stirring 30 minute. Adjust the pH to 5 to 6, raise the temperature to 25-30°C, and add an aqueous solution of sodium chlorite (containing 18 g of sodium chlorite, 0.2 mol) dropwise. After stirring for 3 hours, TLC showed the reaction was complete. After extraction with ethyl acetate, the solvent was removed under reduced pressure to obtain 24 g of solid, with a yield of 82%. H NMR spectrum (CDCl3 1.31(6.3H, s), 1.40(2.7H, s), 1.9 2.11(1H,m), 2.3 2.55(1H, m), 3.3 3.42(1H, m), 3.5 3.65(1H, m), 3.6 3.80(1H, m), 4.37(0.7H, d, J=8.8Hz), 4.47(0.3H, d, J=8.4Hz), 7.1 7.25 (5H, m); ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com