Synthetic method of bis-4-(1H-pyrazol-1-yl) piperidine-1-tert-butyl formate and application thereof
A technology of tert-butyl formate and synthesis method, which is applied in the synthesis of bis-4-piperidine-1-tert-butyl carboxylate and the synthesis of pharmaceutical intermediate impurities, which can solve the problem of increasing the difficulty of final product processing and affecting The purity of Nigerian medicines and other issues can be solved, and the effect of simple method and easy control of the process can be achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 The synthetic method of two-4-(1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert-butyl ester
[0046] The present embodiment is a kind of synthetic method of bis-4-(1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert-butyl ester, which is carried out according to the following steps:
[0047] (11) Mesylation reaction
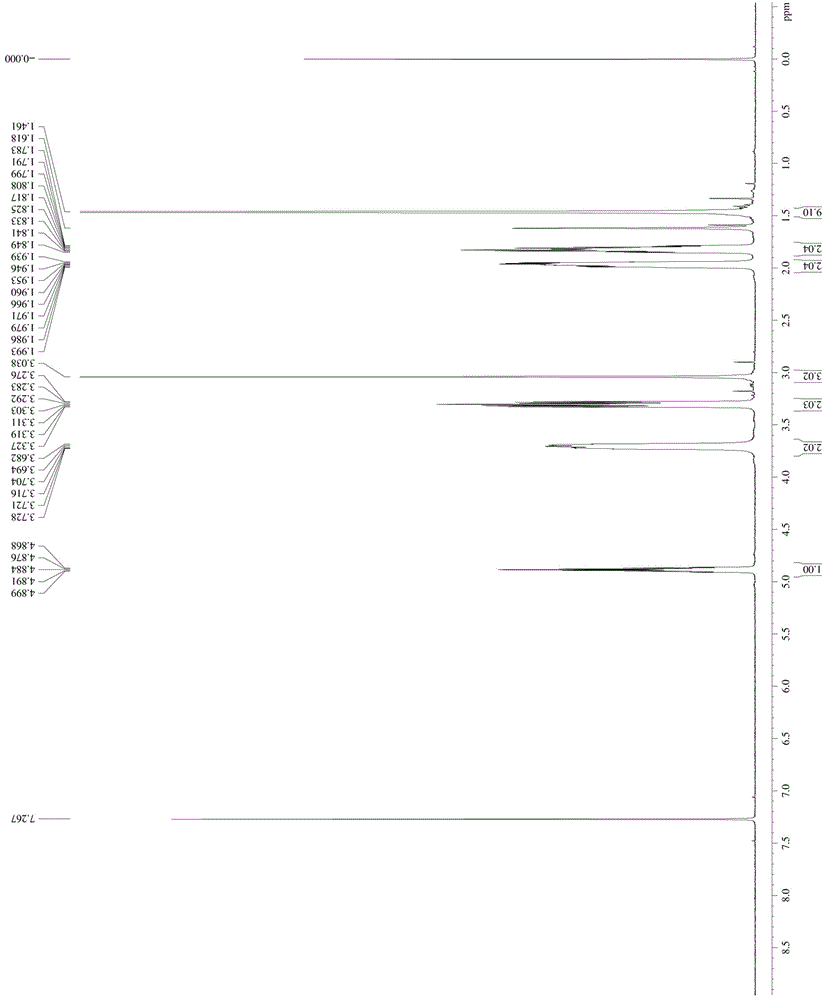
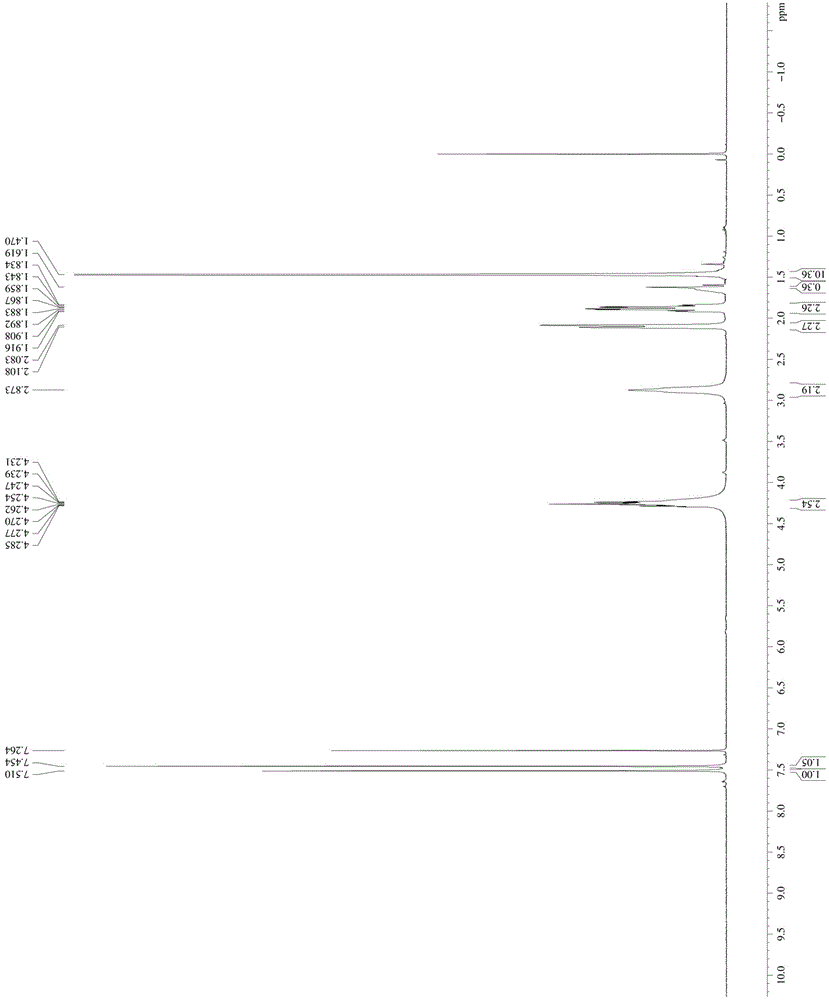
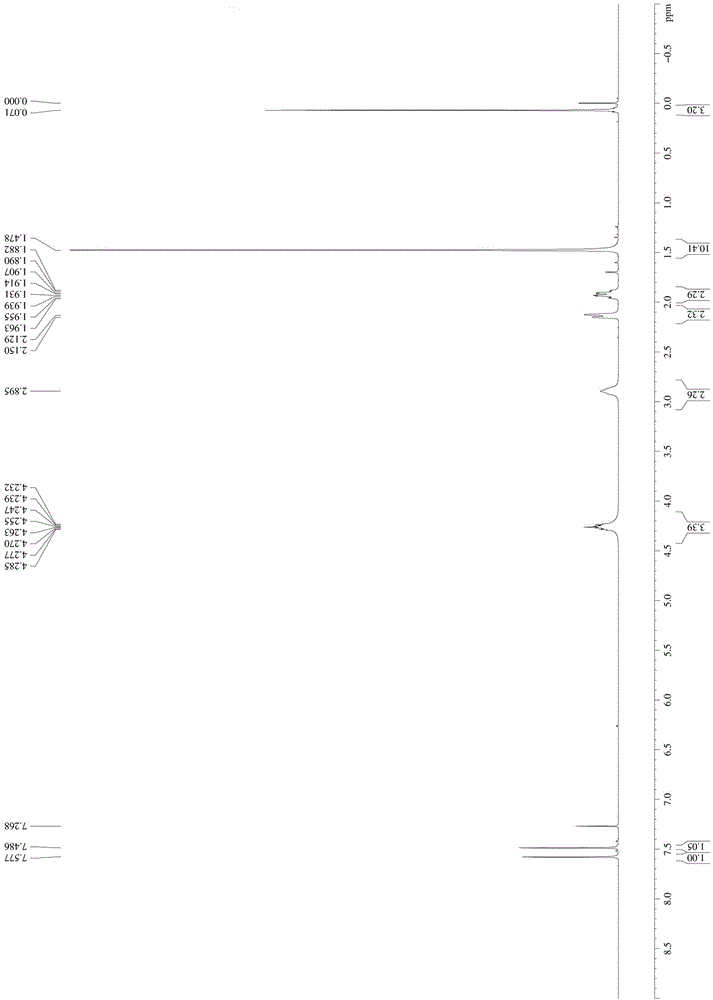
[0048] Take 1mol N-Boc-4-hydroxypiperidine and dissolve it in MTBE / triethylamine mixed solution with a volume ratio of 10:1 at -10°C, add 1.3mol methanesulfonyl chloride (the manufacturer of methanesulfonyl chloride) dropwise Zhengzhou Haorong Chemical Products Co., Ltd.), keep warm for 2 hours after the dropwise addition; add water to quench the reaction, separate the organic phase, add a mixed solution of petroleum ether / n-heptane with a volume ratio of 62:38, and crystallize to obtain compound 1 -Boc-4-methanesulfonyloxypiperidine, denoted as A1;
[0049] The NMR image of A1 is as follows figure 1 As shown, the specific NMR data are as follo...
Embodiment 2-5
[0066] Example 2-5 The synthetic method of two-4-(1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert-butyl ester
[0067] Embodiment 2-5 is respectively a kind of synthetic method of bis-4-(1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert-butyl ester, and their synthetic method is similar to that of Example 1, the difference is only The reason is that the corresponding technical parameters are different during the synthesis process, see the table below for details.
[0068] Table 1 Technical parameter table
[0069]
Embodiment 6
[0070] Example 6 Suzuki coupling reaction condition screening test
[0071] In the process of preparing the target product, in the Suzuki coupling reaction process, the selection of solvent, reaction temperature, reaction time, alkali amount, and phase transfer catalyst dosage are important. In order to explore this condition, the following experiments were carried out in this embodiment:
[0072] Table 2-1 Suzuki coupling reaction condition parameter table
[0073]
[0074] Table 2-2 Parameter table of Suzuki coupling reaction conditions
[0075]
[0076] Table 2-3 Parameter table of Suzuki coupling reaction conditions
[0077]
[0078] Table 2-4 Parameter table of Suzuki coupling reaction conditions
[0079]
[0080] Table 2-5 Parameters of Suzuki coupling reaction conditions
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com