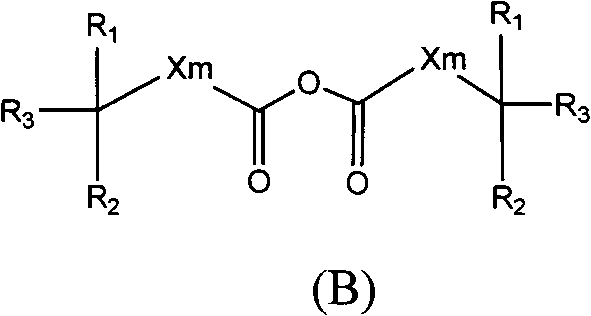
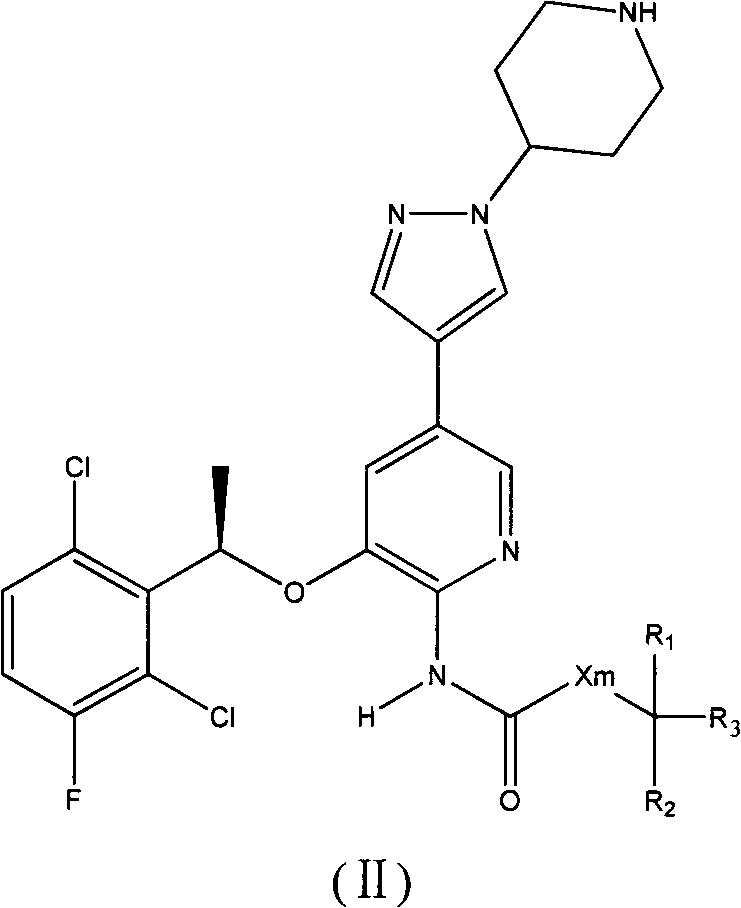
Crizotinib prodrug, as well as preparation and application thereof
A pharmacy and compound technology, applied in the field of organic compound synthesis and medical application, can solve the problem of no pyridine ring amino modification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Preparation of N-acetyl-5-bromo-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-pyridinamine
[0121]
[0122] The compound of this example was prepared by the acid chloride method. Dissolve 500mg of 5-bromo-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-pyridinamine in 15ml of dichloromethane and cool to 0°C After adding 1ml of triethylamine and continuing to stir for 5 minutes, after adding 1.1 equivalent of acetyl chloride dropwise, the temperature was raised to room temperature and reacted for 5 hours. Water was added to terminate the reaction, extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered, and concentrated. The crude product was subjected to column chromatography with ethyl acetate:petroleum ether=1:4 to obtain 400 mg of yellow-white solid with a yield of 72%.
[0123] 1 HNMR (500MHz, CDCl 3 )δ: 8.20(s, 1H), 7.69(d, 1H), 7.34(dd, 1H), 7.10(m, 1H), 6.86(d, 1H), 6.01(q, 1H), 2.47(s, 3H ), 1.84(d, 3H).
Embodiment 2
[0125] Preparation of N-acetyl-5-bromo-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-pyridinamine
[0126]
[0127] The compound of this example was prepared by the acid anhydride method. Add 500mg of 5-bromo-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-pyridinamine into 10ml of acetic anhydride and heat to 50°C for reaction 2 hours. After the reaction, the reaction solution was concentrated to dryness, ethyl acetate and saturated aqueous sodium bicarbonate were added, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated. The crude product was subjected to column chromatography with ethyl acetate:petroleum ether=1:4 to obtain 500 mg of a yellow-white solid with a yield of 90%.
Embodiment 3
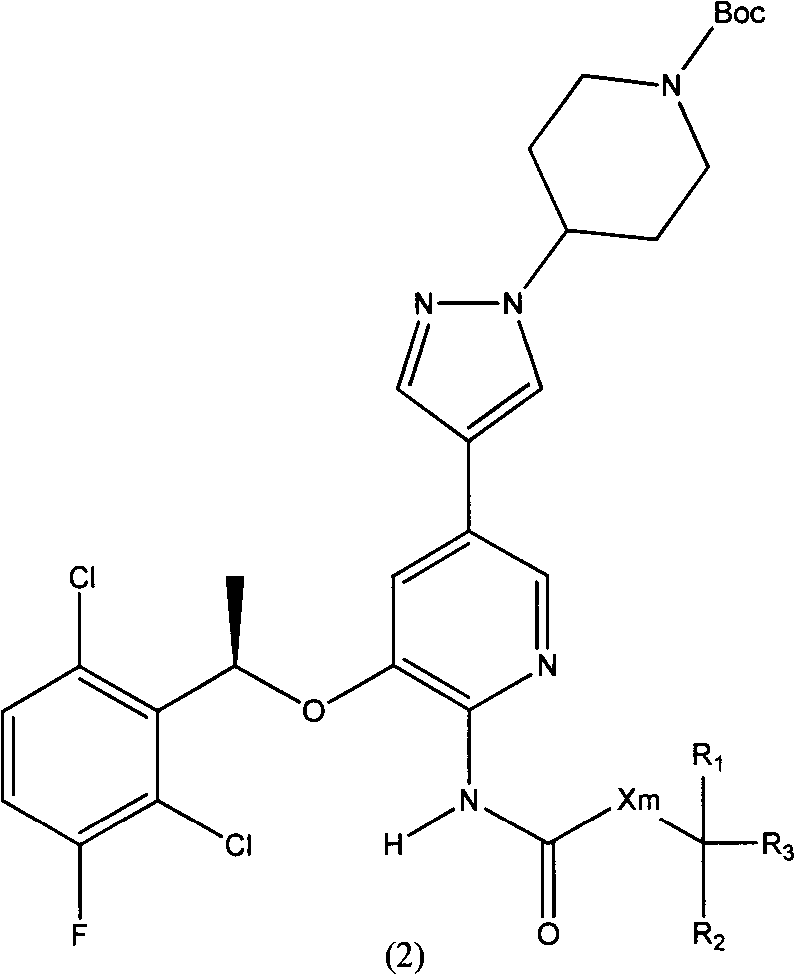
[0129] N-acetyl-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-N-Boc-piperidinyl)- Preparation of 1H-pyrazol-4-yl]-2-pyridinamine
[0130]
[0131] According to the method of Example 1 or Example 2, N-acetyl-5-bromo-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2 - Pyridinamine.
[0132] 300mg N-acetyl-5-bromo-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-pyridinamine and 230mg 1-(4- N-Boc-piperidinyl)-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole (C) In 5ml of DMF, add 300mg of cesium carbonate in 1ml of aqueous solution, replace the air with nitrogen three times, add 20mg of Pd (PPh 3 ) 2 Cl 2 , and then replaced the air with nitrogen three times, and the reaction mixture was warmed to 75° C. and stirred for 12 hours. After the reaction, cool down to room temperature, add 20ml of ethyl acetate to dilute, filter with diatomaceous earth, wash with ethyl acetate, the combined ethyl acetate layer is concentrated after drying with anhydrous sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com