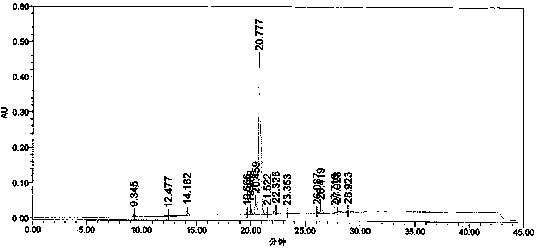
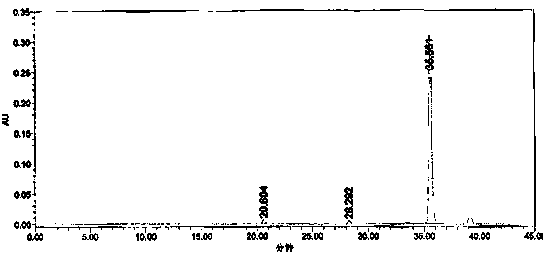
Preparation method for crizotinib
A compound and reaction technology, applied in the field of preparation of crizotinib, can solve the problems of low reaction yield, many reaction by-products, etc., and achieve the effects of short reaction route, shortened reaction period and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of 5-bromo-3-hydroxyl-2-tert-butoxycarbonylaminopyridine
[0050] In 2-amino-3-hydroxy-5-bromopyridine (10.0g, 53.0mmol) and Et 3 N (10mL, 71.8mmol) in dichloromethane (100mL) solution, add Boc 2 O (12.7 g, 58.4 mmol). The reaction was stirred at room temperature for 18 h. Add 150 mL of water to the mixture, continue to stir for 30 min, filter the insoluble matter through celite, separate the organic phase, and extract with 150 mL of dichloromethane. The combined organic phases were washed with saturated NaCl solution (2 × 100 mL), followed by anhydrous NaCl 2 SO 4 dry. The solvent was removed under reduced pressure, EtOAc (200 mL) was added to the residue until dissolved, then activated carbon (2.0 g) was added, and stirred at room temperature for 30 min. Filter through diatomaceous earth, remove the solvent from the filtrate under reduced pressure, add hexane (100 mL) to the residue to make a slurry, filter, and dry in vacuo to obtain 15.0...
Embodiment 2
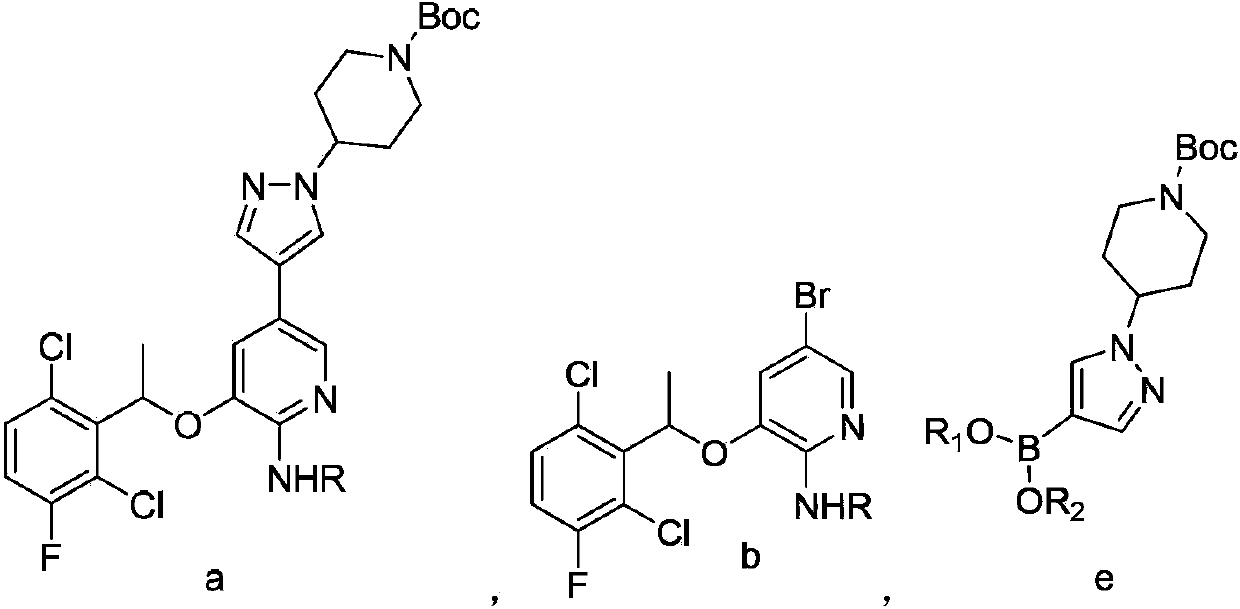
[0052] Example 2: Preparation of 5-bromo-3(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-tert-butoxycarbonylamino-pyridine
[0053] Under nitrogen atmosphere, 1-(2,6-dichloro-3-fluorophenyl)ethanol (1.0g, 4.78mmol), 5-bromo-3-hydroxyl-2-tert-butoxycarbonylaminopyridine (1.4g , 4.78mmol) and triphenylphosphine (1.6g, 6.2mmol) were dissolved in 20mL of anhydrous THF and cooled to below 0°C. Then, diisopropyl azodicarboxylate (1.25 g, 6.2 mmol) was added dropwise, controlling the temperature to <5°C. The mixture was stirred at room temperature for 6 hours. Filter and remove the solvent under reduced pressure to obtain an oily substance, which is recrystallized with ethanol to obtain a white solid 5-bromo-3(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-tert Butoxycarbonylamino-pyridine 2.13g, yield 93.0%.
[0054] 1 H NMR (400MHz, CDCl 3 ):δ8.05(d,J=1.6Hz,1H),7.51(brs,1H),7.32(dd,J=4.8Hz,4.4Hz,1H),7.12-7.08(m,2H),6.05(q ,J=6.4Hz,1H),1.85(d,J=6.4Hz,3H),1.55(s,9H);
Embodiment 3
[0055] Example 3: 3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(1-tert-butoxycarbonyl-piperidin-4-yl)-1H Preparation of -pyrazol-4-yl]-2-tert-butoxycarbonylamino-pyridine
[0056] 5-Bromo-3(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-tert-butoxycarbonylamino-pyridine (0.24g, 0.5mmol) and 4-[4- (4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl]-1-tert-butoxycarbonyl-piperidine (0.19g, 0.5mmol) was dissolved in 5mL DMF, added with Na 2 CO 3 (0.16g, 1.5mmol) in 1mL aqueous solution, add Pd(Ph 3 P) 2 Cl 2 (8.8mg, 0.0125mmol), the reaction mixture was heated to 60°C and stirred for 6 hours under a nitrogen atmosphere, then cooled to room temperature, filtered to remove insoluble matter, and extracted with methyl tert-butyl ether (3×5mL). The combined organic phases were washed with saturated NaCl solution (2 × 5 mL), followed by anhydrous NaCl 2 SO 4 dry. The solvent was removed under reduced pressure to give white solid 3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com