Methane oxidation coupling catalyst and preparation method thereof
A catalyst and methane technology, applied in the direction of catalyst activation/preparation, carbon compound catalyst, catalyst, etc., can solve the problems of easy loss of alkali metal, increased explosion risk, catalyst deactivation, etc., and achieve high thermal and chemical stability, and reaction Good stability and low temperature activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
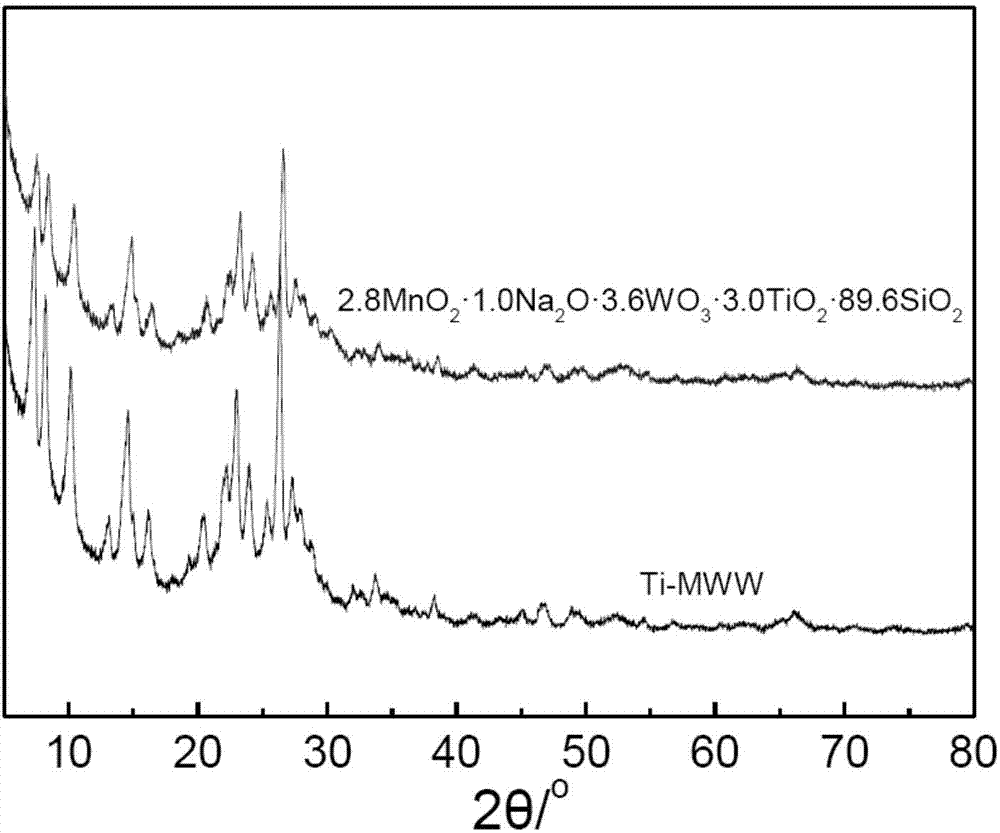
[0042] The purpose of this example is to provide 2.8MnO 2 1.0Na 2 O 3.6 WO 3 3.0TiO 2 89.6SiO2 2 Catalyst preparation.
[0043] a) Weigh 0.11 g of sodium tungstate (Na 2 WO 4 2H 2 O) Dissolve in 2.5ml of deionized water to form an aqueous solution of sodium tungstate; weigh 2.00g of dry Ti-MWW molecular sieve (Si / Ti ratio 40) into a 100ml beaker, first add 5.0ml of deionized water to disperse the molecular sieve Finally, add the sodium tungstate aqueous solution prepared above dropwise, ultrasonically disperse for 0.5 hours and then continue to stir for 3 hours to obtain a slurry-like viscous mixture;
[0044] b) Weigh 0.247 g of 50% manganese nitrate aqueous solution, add deionized water to dilute to 2 ml; add the obtained manganese nitrate aqueous solution dropwise to the slurry-like viscous mixture obtained in step a) under stirring, and continue stirring for 3 hours, Dry at 90°C;
[0045] c) The sample prepared in step b) was ground into powder, and calcined at 55...
Embodiment 2
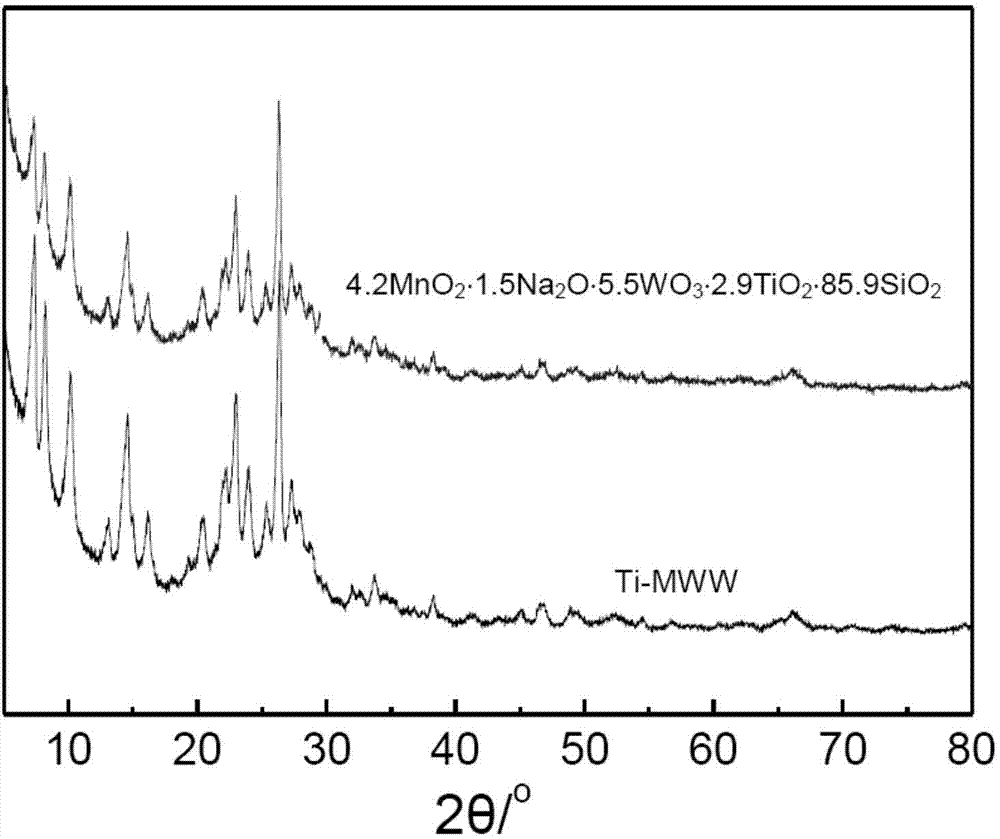
[0048] The purpose of this example is to provide 4.2MnO 2 1.5Na 2 O·5.5WO 3 2.9TiO2 2 ·85.9SiO2 2 Catalyst preparation.
[0049] a) Weigh 0.177 grams of sodium tungstate (Na 2 WO 4 2H 2 O) Dissolve in 2.5ml of deionized water to form an aqueous solution of sodium tungstate; weigh 2.00g of dry Ti-MWW molecular sieve (Si / Ti ratio 40) into a 100ml beaker, first add 5.0ml of deionized water to disperse the molecular sieve Finally, add the sodium tungstate aqueous solution prepared above dropwise, ultrasonically disperse for 1 hour, and then continue stirring for 1 hour to obtain a slurry-like viscous mixture;
[0050] b) Weigh 0.391 g of 50% manganese nitrate aqueous solution, add deionized water to dilute to 2 ml; under stirring, add the obtained manganese nitrate aqueous solution dropwise into the slurry-like viscous mixture obtained in step a), and continue stirring for 1 hour, Dry at 80°C;
[0051] c) The sample prepared in step b) was ground into powder, and calcined...
Embodiment 3
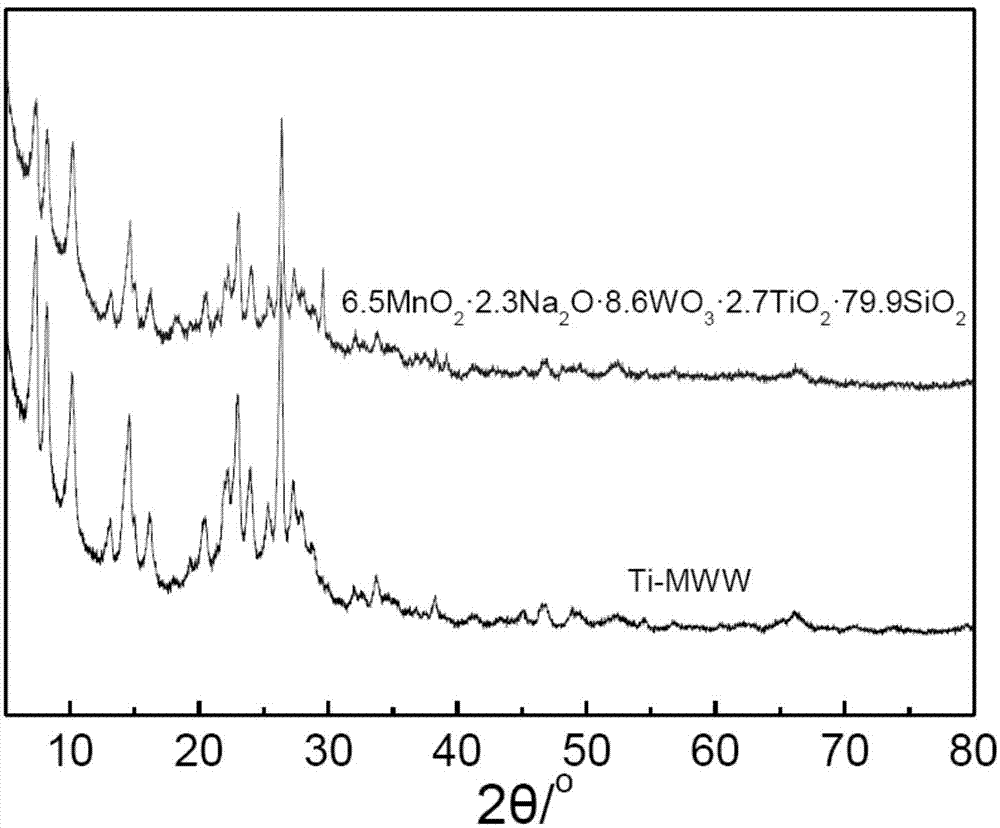
[0054] The purpose of this example is to provide 6.5MnO 2 2.3Na 2 O 8.6 WO 3 2.7TiO2 2 79.9 SiO2 2 Catalyst preparation.
[0055] a) Weigh 0.295 grams of sodium tungstate (Na 2 WO 4 2H 2 O) Dissolve in 2.5ml of deionized water to form an aqueous solution of sodium tungstate; weigh 2.00g of dry Ti-MWW molecular sieve (Si / Ti ratio 40) into a 100ml beaker, first add 5.0ml of deionized water to disperse the molecular sieve Finally, add the sodium tungstate aqueous solution prepared above dropwise, ultrasonically disperse for 0.5 hours, and then continue to stir for 2 hours to obtain a slurry-like viscous mixture;
[0056] b) Weigh 0.651 g of 50% manganese nitrate aqueous solution, add deionized water to dilute to 2 ml; under stirring, add the obtained manganese nitrate aqueous solution dropwise into the slurry-like viscous mixture obtained in step a), and continue stirring for 3 hours, Dry at 100°C;
[0057] c) The sample prepared in step b) was ground into powder, and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com