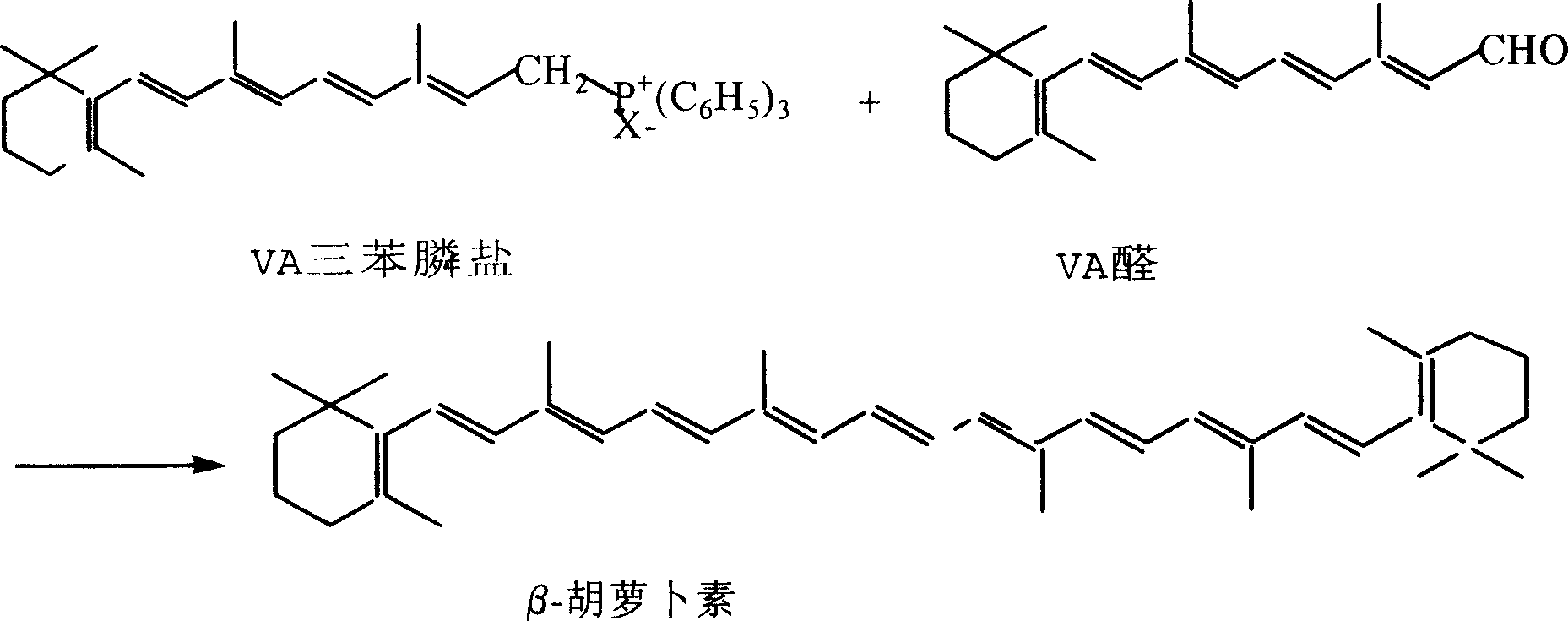
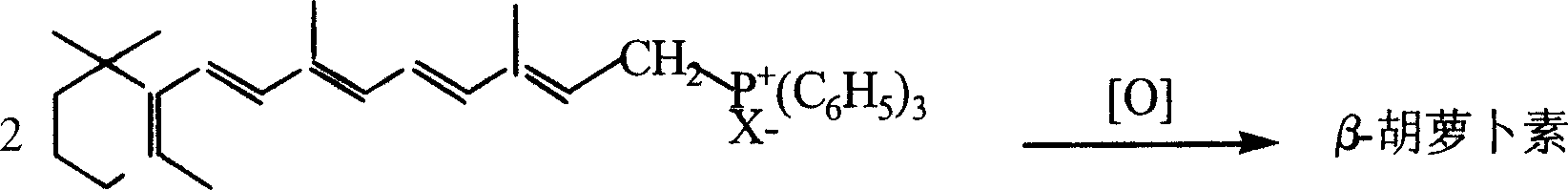Synthesizing technique for beta-carotene
A carotene and synthesis process technology, applied in the direction of organic chemistry, etc., can solve the problems of easy oxidative damage to beta-carotene, difficult to control the feeding ratio, large production fluctuation, etc., to reduce the probability of oxidative damage, and easy to feed the ratio Control, the effect of small production fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of vitamin A triphenylphosphine salt
[0021] Put 50 grams of vitamin A (VA, light yellow crystals, 2.8 million IU, 0.147 moles), 39 grams of triphenylphosphine (0.149 moles) and 500 milliliters of methanol in a 1000 milliliter three-necked bottle, cool to 10 ° C in an ice-water bath under stirring, and maintain for 15 Below ℃, 15 grams of concentrated sulfuric acid was slowly added dropwise, and the addition was completed in about 1 hour, and then continued to keep warm and stirred for 12 hours, and the reaction solution turned into an orange transparent solution. Add 80 grams of deionized water, extract twice with 100 milliliters of n-hexane each time, the lower layer is the methanol-water solution of VA triphenylphosphine salt, and recover the solvent methanol under reduced pressure below 30 ° C until solids are precipitated (at this time, the mixed system Weighing 130 grams), then 600 grams of water was added to dissolve the solid into a cl...
Embodiment 2
[0022] Embodiment 2: Coupling reaction prepares β-carotene
[0023] Put the aqueous solution of VA triphenylphosphine salt obtained in Example 1 in a 2000 ml four-necked bottle, add 600 ml of chloroform, cool to 0°C in an ice-salt bath, and add dropwise sodium hypochlorite containing 10% available chlorine under 5°C 150 grams of aqueous solution, while also adding saturated aqueous sodium carbonate dropwise to ensure that the pH value is between 8-10 after the reaction is completed, the addition is completed in about 1 hour, and the insulation is continued to stir for 1 hour.
[0024] Separate the layers to obtain a red organic layer, wash it with 200 ml of water, recover the solvent below 55°C, and obtain a red fine powder, add 200 ml of methanol, reflux for 10 minutes, filter to obtain 68 g of crude product β-carotene, and weigh it after vacuum drying. 61.2 grams, the content detection is 68%. The crude product was refluxed in n-heptane for 12 hours under the protection of ...
Embodiment 3
[0025] Embodiment 3: prepare β-carotene with VA crystallization mother liquor
[0026] Be the VA crystallization mother liquor of 1.3 million IU with 110 gram content (liquid chromatography analysis: all-trans VA acetate 42%; 13-cis VA acetate 40%, trans VA alcohol 13%) replace embodiment 1 VA crystal in the same other conditions, the aqueous solution of VA triphenylphosphine salt was obtained.
[0027] Put the aqueous solution of VA triphenylphosphine salt in a 2000 ml four-necked bottle, add 600 ml of chloroform, cool in an ice-salt bath to 0°C, keep it below 5°C, add 30 grams of 30% hydrogen peroxide, and then keep it below 10°C Add saturated sodium carbonate aqueous solution dropwise until the pH value of the system is greater than 9, the addition is completed in about 1 hour, and the temperature is kept and the stirring is continued for 1 hour.
[0028] Separate the layers to obtain a red organic layer, wash it with 200 ml of water, recover the solvent below 55 ° C, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com