Preparation method of crizotinib intermediate
A technology for crizotinib and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of long process flow, high production cost, troublesome post-processing, etc., and achieves simple and safe process operation, good application prospect, and easy purchase. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
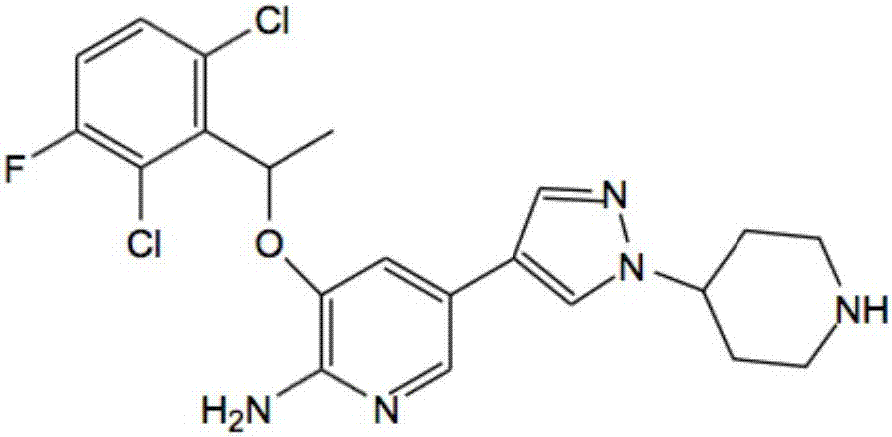
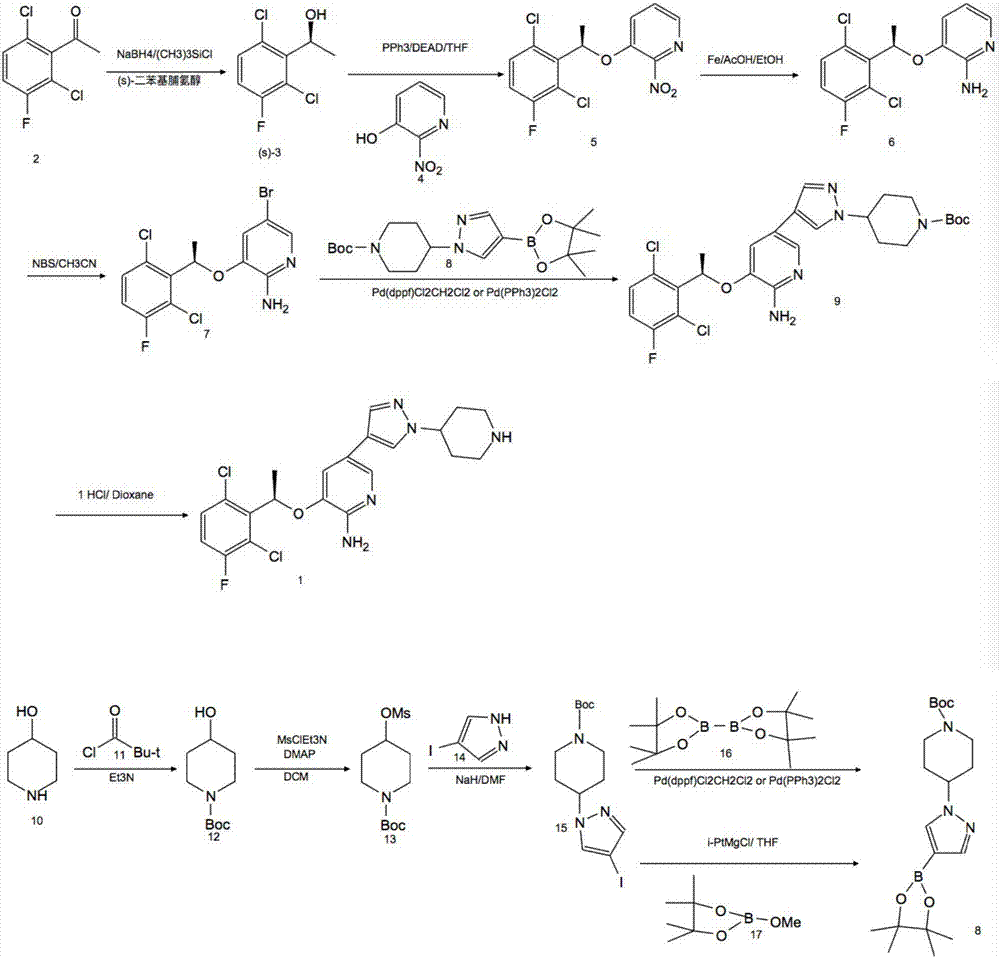
[0027] Compound (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-nitropyridine, sodium dithionate, sodium hydroxide and water in molar ratio 1:1:3:5, put it in the stainless steel reactor of the ball mill, react for 1h, take out the reaction mixture, add 2 times of water to stir, filter to get (R)-3-[1-(2,6-dichloro -3-fluorophenyl)ethoxy]-2-aminopyridine (1), 90% yield by HPLC analysis.
[0028] Compound (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-nitropyridine was dissolved in industrial alcohol, 1%Pd / C catalyst was added, and Temperature 20°C, H 2 React under the condition of pressure 0.1MPa. After the reaction is complete, filter, add water, extract with dichloromethane, and concentrate to obtain (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-aminopyridine (1) , HPLC analysis yield 95%.
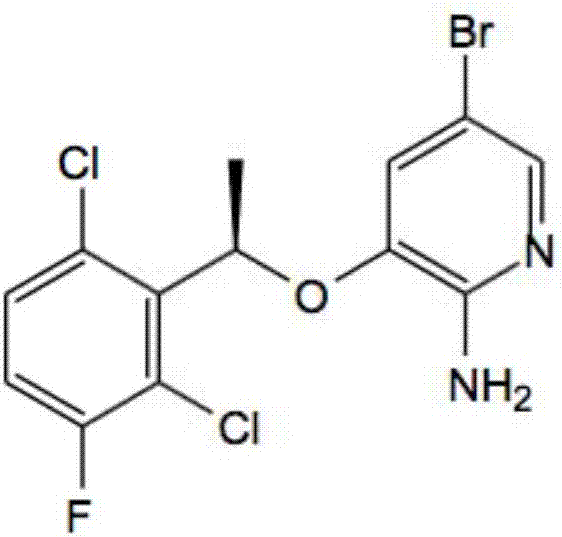
[0029] Potassium persulfate and sodium bromide molar ratio of 1:1 was prepared as an aqueous solution, placed in a dry three-necked flask, T=15°C, compound (1) was dissolved in ...
Embodiment 2
[0031] Compound (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-nitropyridine, sodium dithionate, sodium hydroxide and water in molar ratio 1:2:4:8, placed in a stainless steel reactor of a ball mill, reacted for 2 hours, took out the reaction mixture, added 2 times of water for stirring, and filtered to obtain (R)-3-[1-(2,6-dichloro -3-fluorophenyl)ethoxy]-2-aminopyridine (1), the yield by HPLC analysis was 89%.
[0032] Dissolve compound (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-nitropyridine in methanol, add 2% Pt / C catalyst, at temperature 30°C, H 2 React under the condition of pressure 0.2MPa. After the reaction is complete, filter, add water, extract with dichloromethane, and concentrate to obtain (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-aminopyridine (1) , HPLC analysis yield 85%.
[0033] The molar ratio of potassium persulfate to sodium bromide was 1:1.5 to prepare an aqueous solution, which was placed in a dry three-necked flask at T=20°C. Compound ...
Embodiment 3
[0035] Compound (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxyl]-2-nitropyridine, sodium dithionate, sodium hydroxide and water in molar ratio 1:3:5:5, put it in the stainless steel reactor of the ball mill, react for 1h, take out the reaction mixture, add 2 times of water to stir, filter to get (R)-3-[1-(2,6-dichloro -3-fluorophenyl)ethoxy]-2-aminopyridine (1), the yield by HPLC analysis was 86%.
[0036] The compound (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-nitropyridine was dissolved in methanol and 2% Pd / Al was added 2 o 3 catalyst, at a temperature of 30 °C, H 2 React under the condition of pressure 0.2MPa. After the reaction is complete, filter, add water, extract with dichloromethane, and concentrate to obtain (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-aminopyridine (1) , HPLC analysis yield 89%.
[0037]Potassium persulfate and sodium bromide molar ratio of 1:1.5 was prepared as an aqueous solution, placed in a dry three-necked flask, T=15°C, compound (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com