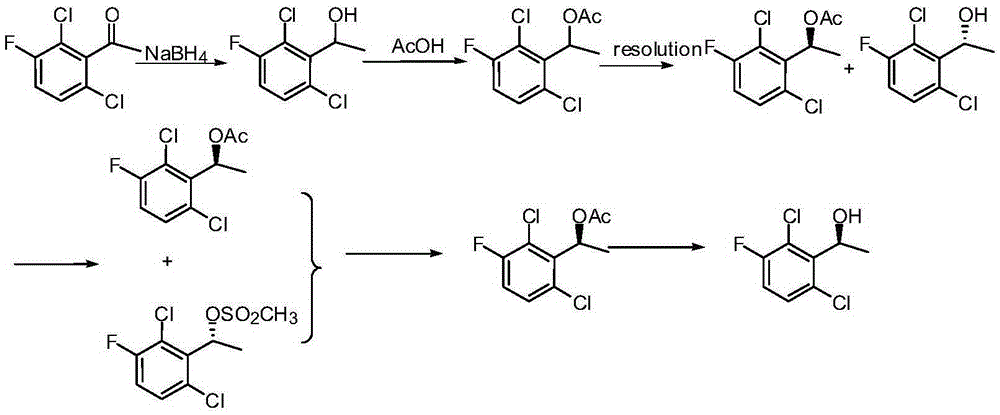
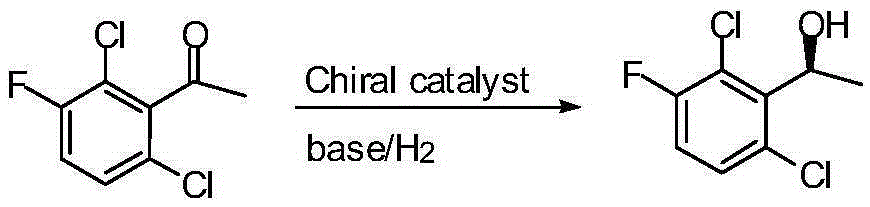
Preparation method of crizotinib intermediate (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol
A crizotinib and intermediate technology, which is applied in the field of preparation of crizotinib intermediate-1-ethanol, can solve the problems of expensive catalyst, time-consuming separation and purification, and high product cost, and saves the cost of process raw materials, Reduced handling and material loss, high optical content effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
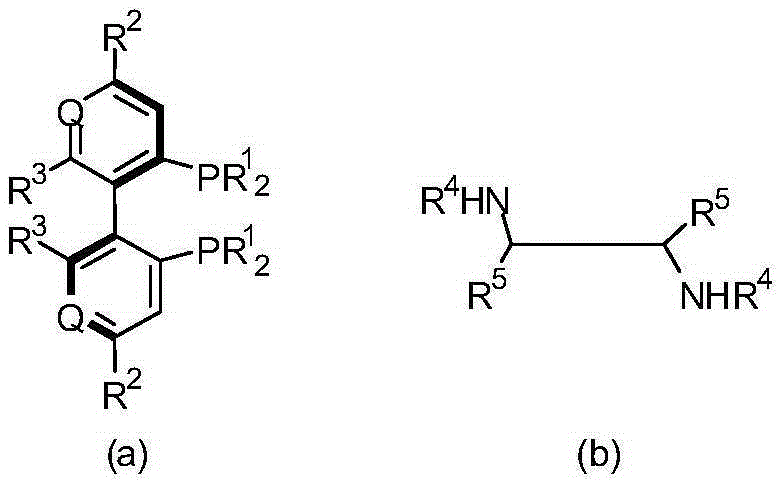
[0029] Weigh 1.9 mg, 0.002 mmol of chiral catalyst [RuCl 2 ((S)-P-Phos)((R,R)-DPEN)]; 224 mg, 2.0 mmol of potassium tert-butoxide; 30 mL of isopropanol; 14.8 mL, 0.1 mol of 1-(2,6-dichloro- 3-fluorophenyl)ethanone. The chiral catalyst consists of RuCl 2 (p-cymene), (R,R)-DPEN and (S)-P-Phos coordination combination, the RuCl 2 (p-cymene) and (R,R)-DPEN are from Reagent Company, (S)-P-Phos is from Wuhan Shenxinda Chemical Technology Co., Ltd. Potassium tert-butoxide, isopropanol, 1-(2,6 -Dichloro-3-fluorophenyl)ethanone is chemically pure purchased from a reagent company. Put the above-mentioned chiral catalyst, potassium tert-butoxide, isopropanol and 1-(2,6-dichloro-3-fluorophenyl)ethanone in the reaction tube and mix thoroughly, then put the reaction tube into the autoclave , the upper end of the reaction tube is open, and the gas in the kettle body is replaced with nitrogen and hydrogen respectively, so that the hydrogen pressure reaches 2MPa, the temperature is raised ...
Embodiment 2
[0031]Weigh 1.9 mg, 0.002 mmol of chiral catalyst [RuCl 2 ((S)-P-Phos)((R,R)-DPEN)]; 98 mg, 2.0 mmol sodium amide; 30 mL of propanol; 14.8 mL, 0.1 mol 1-(2,6-dichloro-3-fluorobenzene base) ethyl ketone. The chiral catalyst consists of RuCl 2 (p-cymene), (R,R)-DPEN and (S)-P-Phos coordination combination, the RuCl 2 (p-cymene) and (R,R)-DPEN are from reagent companies, (S)-P-Phos is from Wuhan Shenxinda Chemical Technology Co., Ltd., sodium amide, propanol, 1-(2,6-dichloro -3-fluorophenyl)ethanone is chemically pure purchased from a reagent company. The above-mentioned chiral catalyst, sodium amide, propanol and 1-(2,6-dichloro-3-fluorophenyl)ethanone are placed in a reaction tube and fully mixed, and the reaction tube is placed in a high-pressure reactor. Open the upper end of the reaction tube, replace the gas in the kettle with nitrogen and hydrogen respectively, make the hydrogen pressure reach 4MPa, raise the temperature to 80°C and keep it warm for more than 5 hours u...
Embodiment 3
[0033] Weigh 2.3 mg, about 0.002 mmol of chiral catalyst [RuCl 2 ((S)-P-Phos)((R,R)-DPEN)]; 136 mg, 2.0 mmol sodium ethoxide; 50 mL of THF; 14.8 mL, 0.1 mol 1-(2,6-dichloro-3-fluorophenyl ) ethyl ketone. The chiral catalyst consists of RuCl 2 (p-cymene), (R,R)-DPEN and (S)-P-Phos coordination combination, the RuCl 2 (p-cymene) and (R,R)-DPEN are from reagent companies, (S)-P-Phos is from Wuhan Shenxinda Chemical Technology Co., Ltd., sodium ethoxide, tetrahydrofuran, 1-(2,6-dichloro- All 3-fluorophenyl)ethanone was chemically pure purchased from a reagent company. The above-mentioned chiral catalyst, potassium tert-butoxide, tetrahydrofuran and 1-(2,6-dichloro-3-fluorophenyl)ethanone are placed in a reaction tube and fully mixed, and the reaction tube is placed in a high-pressure reactor. The upper end of the reaction tube is open, replace the gas in the kettle body with nitrogen and hydrogen respectively, make the hydrogen pressure reach 5MPa, raise the temperature to 50°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com