Preparation method of crizotinib
A crizotinib and pyrazolyl technology, applied in the field of preparation of crizotinib, can solve the problems of difficult separation, difficult industrial production, boron ester heat and acid instability, etc., and achieves good optical purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1, the preparation of boronic acid compound (3)
[0042]
[0043] Add 4L of freshly steamed tetrahydrofuran into a 50L kettle, add 2.60Kg of 4-iodopyrazole compound (11), dissolve and add 10L of isopropylmagnesium chloride solution (3.0M) dropwise, and control the reaction temperature below 25°C. After the dropwise addition was completed, the mixture was stirred at room temperature for 12 hours. The completion of the reaction was monitored by TLC, and 3 L of tetrahydrofuran solution in which 2.34 Kg of trimethoxyborane (21) was dissolved was added dropwise to the reaction solution. Control the reaction temperature at about 25°C, and continue stirring for 12 hours after the dropwise addition is completed. After the completion of the reaction monitored by TLC, the reaction was quenched with saturated aqueous ammonium chloride solution. Ethyl acetate was added, the layers were separated, and the organic layer was taken. The organic layer was washed successively with ...
Embodiment 2
[0051] 1, the preparation of boronic acid compound (3)
[0052]
[0053] Add 4L of freshly steamed tetrahydrofuran into a 50L kettle, add 1.9Kg of 4-bromopyrazole compound (12), dissolve and add 10L of isopropylmagnesium chloride solution (3.0M) dropwise. Stir at room temperature for 12 hours. After TLC monitored the completion of the reaction, 3 L of a tetrahydrofuran solution of 1.2 Kg of methoxyboronic acid pinacol ester (22) was added dropwise. After the dropwise addition was complete, stirring was continued for 12 hours. After the completion of the reaction monitored by TLC, it was quenched with saturated aqueous ammonium chloride. Ethyl acetate was added, and the layers were separated; the organic layer was washed with saturated aqueous sodium bicarbonate and water, and then concentrated to obtain 1.8Kg of solid.
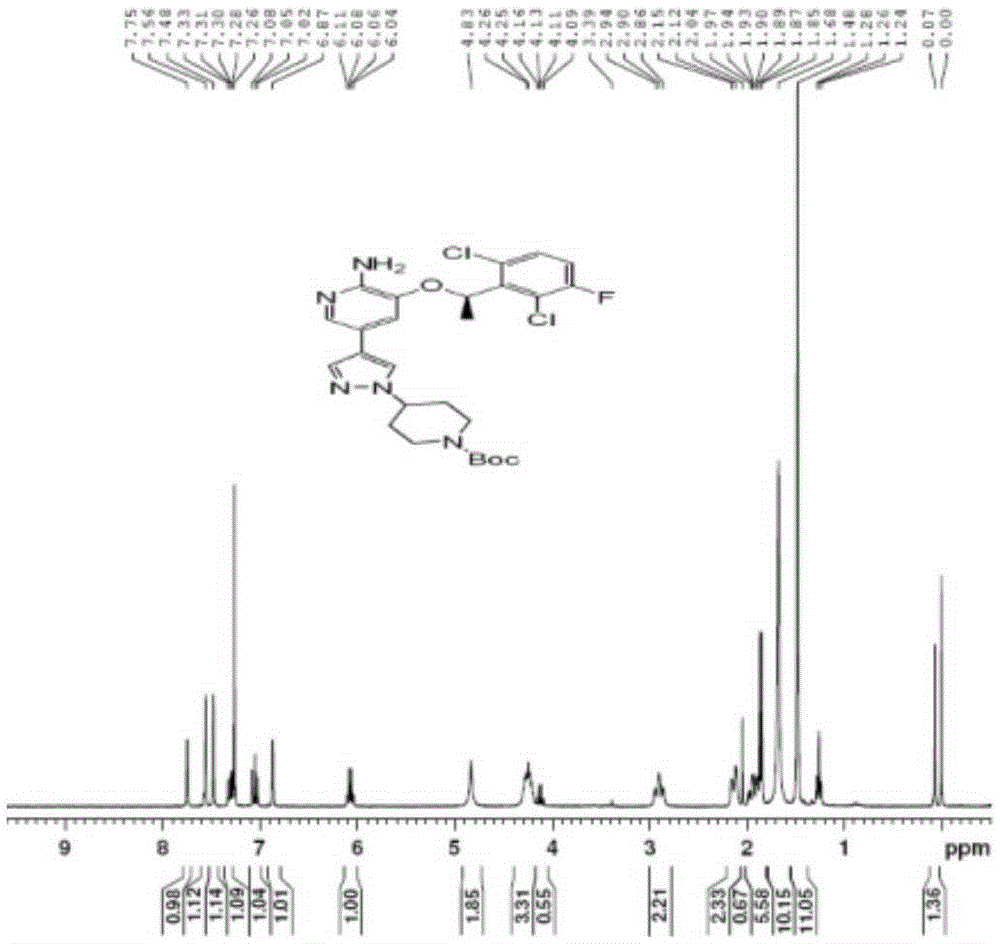
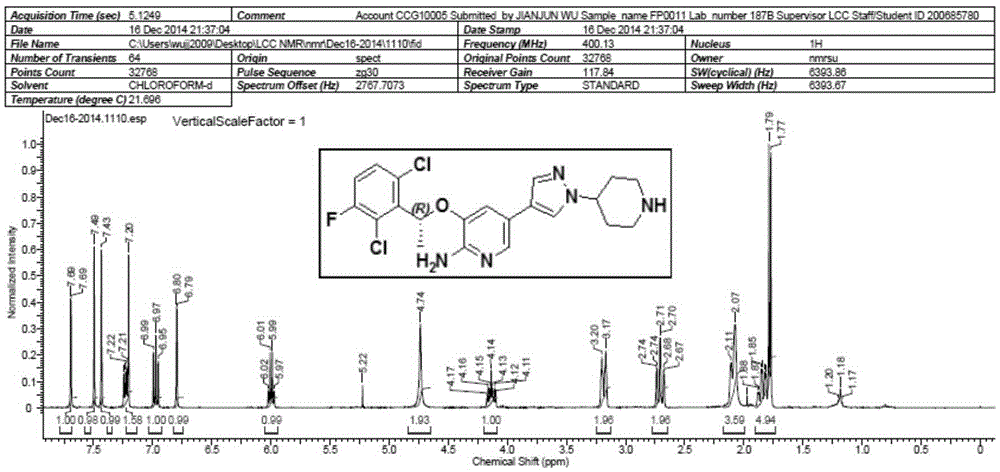
[0054] 2. Preparation of Boc-crizotinib (5)
[0055]
[0056] Add 200g of boric acid compound (3), 100g of brominated compound (4) and 2g of phase t...
Embodiment 3
[0061] 1, the preparation of boronic acid compound (3)
[0062]
[0063] Add 4L of freshly steamed tetrahydrofuran into a 50L kettle, add 2.60Kg of 4-iodopyrazole compound (11), dissolve and add 10L of isopropylmagnesium chloride solution (3.0M) dropwise. Stir at room temperature for 12 hours. After TLC monitored the completion of the reaction, 3 L of tetrahydrofuran solution of 2.34 Kg isopropoxy pinacol borate (23) was added dropwise. After the dropwise addition was complete, stirring was continued for 12 hours. After the completion of the reaction monitored by TLC, it was quenched with saturated aqueous ammonium chloride. Ethyl acetate was added, and the layers were separated; the organic layer was washed with saturated aqueous sodium bicarbonate and water, and then concentrated to obtain 2.7Kg of solid.
[0064] 2. Preparation of Boc-crizotinib (5)
[0065]
[0066] Add 200g of boric acid compound (3), 100g of brominated compound (4) and 2g of phase transfer cata...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com