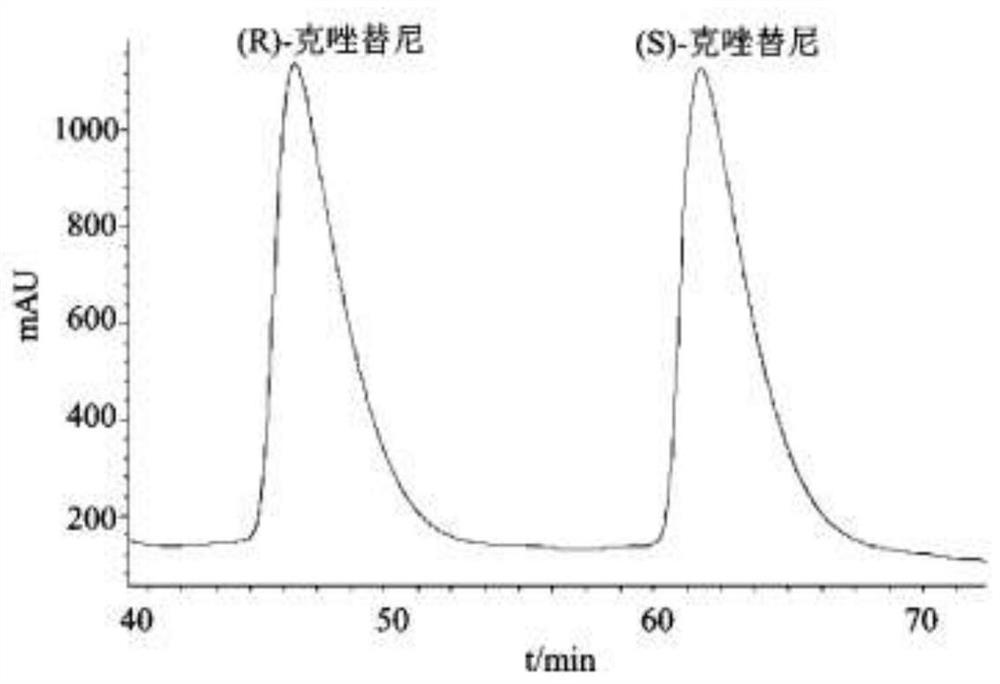
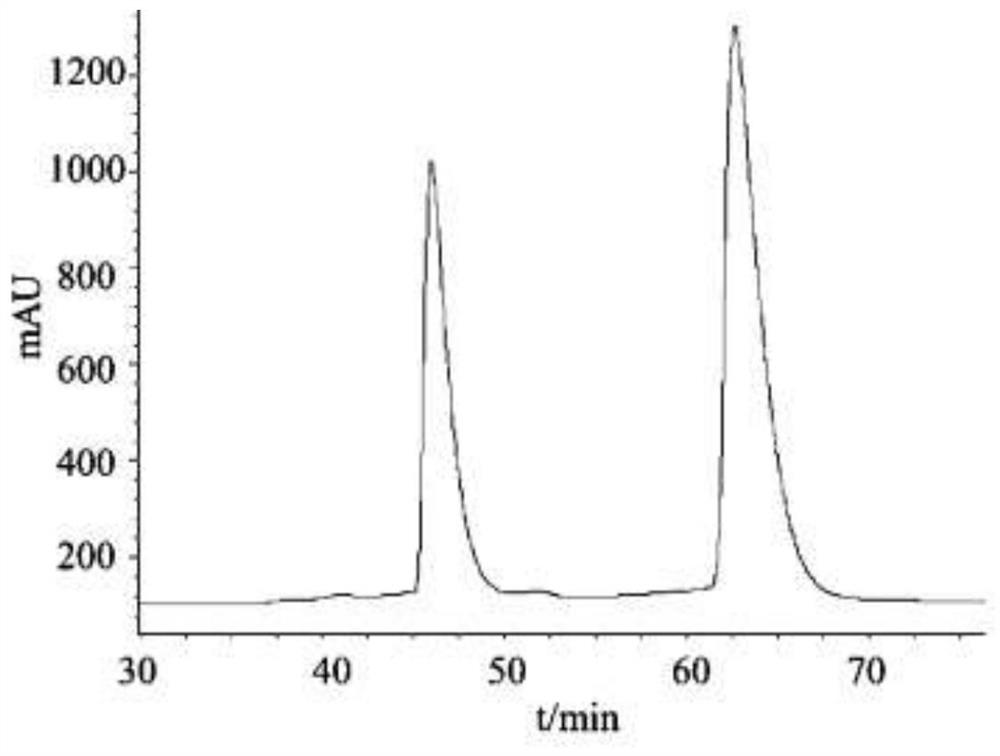
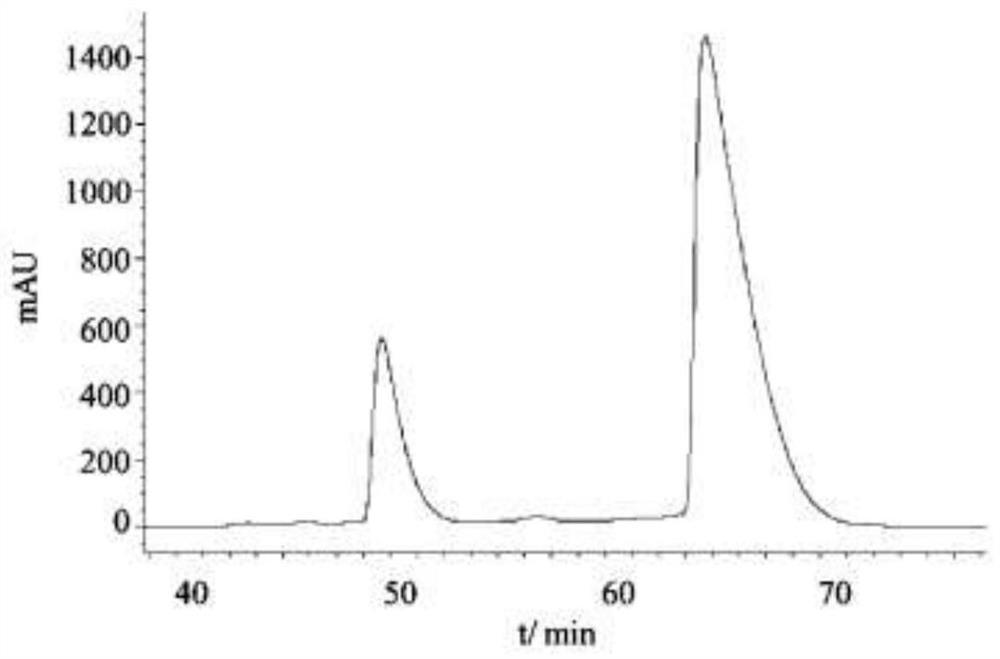
Method for resolving racemic crizotinib
A technology of crizotinib and crizotinib, applied in the field of splitting racemic crizotinib, which can solve the problems of not being able to obtain optically pure single enantiomer, wide metastable zone width, and difficulty in nucleation , to achieve good selective adsorption performance, mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A kind of preparation method of adsorbent, concrete steps are as follows:
[0060] (1) Preparation of MIL-53-NH 2 Nanocrystals: 0.76g AlCl 3 ·6H 2 O was dissolved in 15mL DMF, sonicated for 30min to obtain solution A, and then 0.56g NH 2 -H 2 Dissolve BDC in 15mL DMF, sonicate for 30min to obtain solution B, then add solution A dropwise to solution B at a rate of 1mL / min to obtain a mixed solution, transfer the mixed solution to a 100mL polytetrafluoroethylene reaction In the still, react at 150°C for 24h, and then centrifuge to remove the liquid component at 8500rpm for 8min, activate the obtained solid substance in DMF for 24h, wash with methanol for 3 times, and then remove the liquid component at 120°C After drying for 12 hours, the yellow powder MIL-53-NH was finally obtained 2 Nano crystals; wherein the amount of DMF added during activation meets the 4cm of the solid material interface;
[0061] (2) Dissolve 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydroc...
Embodiment 2
[0065] A kind of preparation method of adsorbent, concrete steps are as follows:
[0066] (1) Preparation of MIL-53-NH 2 Nanocrystals: 0.25g AlCl 3 ·6H 2 O was dissolved in 10mL DMF, sonicated for 25min to obtain solution A, and then 0.19g NH 2 -H 2 Dissolve BDC in 15mL DMF, sonicate for 25min to obtain solution B, then add solution A to solution B dropwise at a rate of 1mL / min to obtain a mixed solution, transfer the mixed solution to a polytetrafluoroethylene with a volume of 100mL Lined reaction kettle, reacted at 150°C for 24h, then centrifuged to remove the liquid component at 8500rpm, centrifuged for 8min, activated the obtained solid substance in DMF for 24h, washed 4 times with methanol, and then placed in Dry at 120°C for 15 hours to finally obtain MIL-53-NH in the form of yellow powder 2 Nano crystals; wherein the amount of DMF added during activation meets the 2cm of the solid material interface;
[0067] (2) Dissolve 1-ethyl-(3-dimethylaminopropyl)carbodiimid...
Embodiment 3
[0071] A kind of preparation method of adsorbent, concrete steps are as follows:
[0072] (1) Preparation of MIL-53-NH 2 Nanocrystals: 0.5g AlCl 3 ·6H 2 O was dissolved in 10mL DMF, sonicated for 26min to obtain solution A, and then 0.25g NH 2 -H 2Dissolve BDC in 15mL DMF, sonicate for 26min to obtain solution B, then add solution A to solution B dropwise at a rate of 1mL / min to obtain a mixed solution, transfer the mixed solution to a polytetrafluoroethylene with a volume of 100mL Lined reaction kettle, reacted at 150°C for 28h, then centrifuged to remove the liquid component at 8500rpm, centrifuged for 8min, activated the obtained solid substance in DMF for 24h, washed 5 times with methanol, and then Dry at 120°C for 18 hours to finally obtain MIL-53-NH in the form of yellow powder 2 Nano crystals; wherein the amount of DMF added during activation meets the 2cm of the solid material interface;
[0073] (2) Dissolve 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com