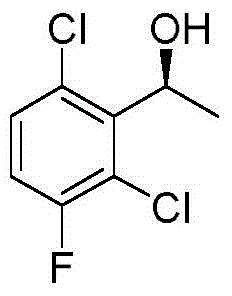
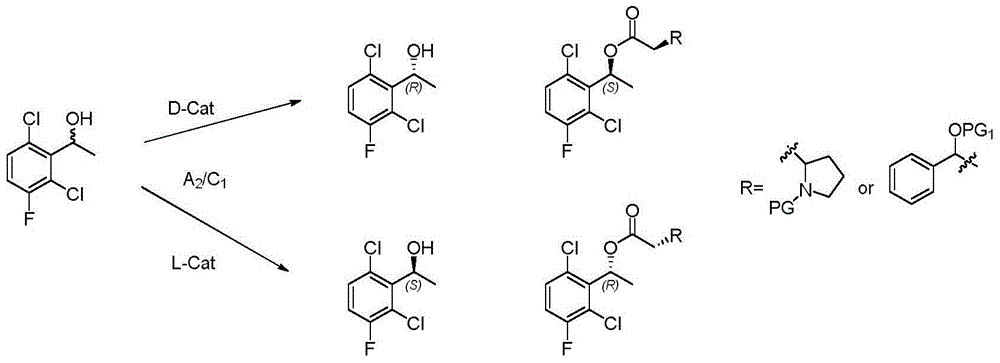
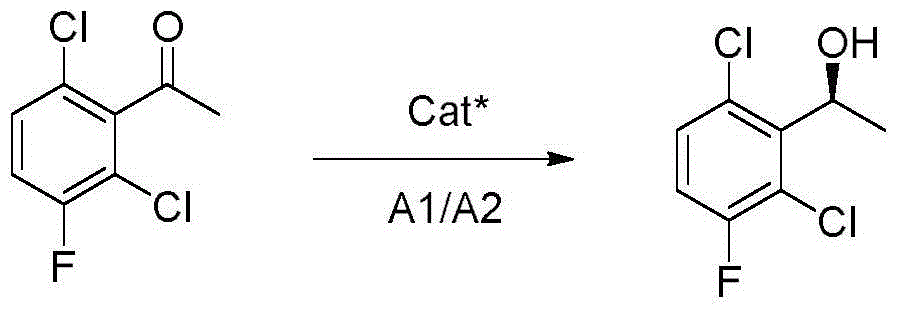
Synthetic method for crizotinib intermediate
A technology of crizotinib and a synthetic method, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of difficult large-scale industrial production, expensive enzyme catalysts, harsh reaction conditions, etc., and achieve good application prospects, easy purchase, Quality Controlled Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add compound (I) 2,6-dichloro-3-fluoroacetophenone (100g, 0.488mol, 1eq), tetrahydrofuran (10L) to the dry reaction flask, start stirring, cool to 0 degrees Celsius under nitrogen protection, drop (-)DIP-Cl (60% W / W n-heptane solution, 651.5g, 1.22mol, 2.5eq), the dropwise addition was completed within 90 minutes, slowly raised to 25 degrees Celsius, stirred for 15 hours, followed by HPLC, the reaction was complete, 0 degrees Celsius Slowly add saturated sodium bicarbonate solution, adjust the pH to about 7, let the layers stand still, and evaporate the organic solvent. After the residue is cooled to room temperature, add 6L of n-hexane, stir at room temperature for 1 hour, place in the refrigerator overnight, and filter while cold. The filter cake was washed with 1 L of iced n-hexane to obtain a white solid, crizotinib intermediate compound (II) (95 g, yield 94%, ee98%).
Embodiment 2
[0025] Add compound (I) 2,6-dichloro-3-fluoroacetophenone (100g, 0.488mol, 1eq), tetrahydrofuran (10L) to the dry reaction flask, start stirring, cool to 0 degrees Celsius under nitrogen protection, drop (-)DIP-Cl (60% W / W n-heptane solution, 391g, 0.73mol, 1.5eq), the dropwise addition was completed within 60 minutes, slowly raised to 25 degrees Celsius, stirred for 15 hours, the reaction was complete after HPLC tracking, and the reaction was slow at 0 degrees Celsius Slowly add saturated sodium bicarbonate solution, adjust the pH to about 7, let the layers stand still, and evaporate the organic solvent. After the residue is cooled to room temperature, add 6L of n-hexane, stir at room temperature for 1 hour, place in the refrigerator overnight, and filter while cold. The cake was washed with 1 L of iced n-hexane to obtain a white solid, crizotinib intermediate compound (II) (75 g, yield 74%, ee88%).
Embodiment 3
[0027] Add compound (I) 2,6-dichloro-3-fluoroacetophenone (100g, 0.488mol, 1eq), tetrahydrofuran (10L) to the dry reaction flask, start stirring, cool to 0 degrees Celsius under nitrogen protection, drop (-)DIP-Cl (60% W / W n-heptane solution, 781.8g, 1.46mol, 3eq), the dropwise addition was completed within 90 minutes, slowly raised to 25 degrees Celsius, stirred for 15 hours, the reaction was complete after HPLC tracking, and the reaction was slow at 0 degrees Celsius Slowly add saturated sodium bicarbonate solution, adjust the pH to about 7, let the layers stand still, and evaporate the organic solvent. After the residue is cooled to room temperature, add 6L of n-hexane, stir at room temperature for 1 hour, place in the refrigerator overnight, and filter while cold. The cake was washed with 1 L of iced n-hexane to obtain a white solid, crizotinib intermediate compound (II) (90 g, yield 89%, ee93%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com