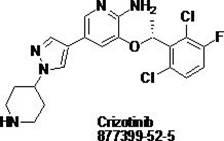
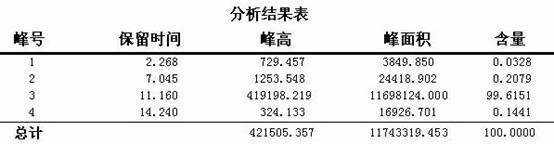
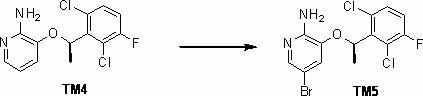Synthesis method of crizotinib serving as antitumor molecular targeting medicament
A technology for crizotinib and anti-tumor molecules, which is applied in the field of new splitting process and the synthesis of intermediates, and can solve the problems of unsatisfactory yield and purity, complicated steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0065] Example 1: Synthesis of racemic 1-(2,6-dichloro-3-fluorophenyl)ethanol (TM1)
[0066] In a 5 L three-necked flask, 832 grams of SM1 was added to a mixed solvent of 1.5 L of ethanol and 1.5 L of THF, the solution was heated to 35°C, and 182 grams of sodium borohydride was added, the reaction was exothermic, and bubbles were generated, and the reaction was carried out for 2 hours Then add 50 g of sodium borohydride, and TLC detects that the reaction is complete in 2 hours (PE / EA=10:1). Add 200 g of water to the system, stir for half an hour, a large amount of solids appear, remove the solvent under reduced pressure, add water and ethyl acetate for extraction, wash the ethyl acetate layer with salt, dry and spin dry to obtain 800 g of product TM1, the yield is 97%. 1 H NMR (400 MHz, DMSO-d 6 ) δppm 7.42 (m, 1H), 7.32 (m, 1H), 5.42 (m, 2H), 1.45 (d, J = 6.4 Hz, 3H).
example 2
[0067] Example 2: Preparation of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol (TM2)
[0068] 500 g of TM1, 322 g of Boc-L-proline were dissolved in 1.5 L of anhydrous dichloromethane, and 2.5 L of a dichloromethane solution of 375 g of EDCI and 118 g of p-toluenesulfonic acid was added dropwise at 0°C. After the completion of the reaction at room temperature for 2 hours, the completion of the reaction was monitored by TLC (PE / EA=5:1). The reaction solution was washed with water, dried, and evaporated to dryness, and the fraction was collected at 100-110°C under a vacuum of 10 mm Hg with an oil pump to obtain a crude oily product, which was dissolved in 250 mL of n-hexane at room temperature, and the temperature was lowered to -20°C. Precipitated, and filtered at low temperature to obtain 150 g of white solid with a yield of 60% (ee%>98%).
[0069] HPLC detection conditions (n-hexane / isopropanol=99.3:0.7, flow rate 1.5 mL / s, peak elution time 8-9 min). HPLC instrument: Shimadzu...
example 3
[0073] Example 3: Synthesis of (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-nitropyridine (TM3)
[0074] 300 grams of TM2, 205 grams of SM2 and 540 grams of triphenylphosphine were dissolved in 2L THF, and 361 grams of DEAD was added dropwise at 0°C. After the addition was completed, the mixture was stirred at room temperature for two hours, and the reaction was detected by TLC. (PE / EA=3:1). Remove THF by rotary evaporation, add water, extract with ethyl acetate 1 Lx3, wash the ethyl acetate layer with saturated aqueous sodium bicarbonate solution, dry the ethyl acetate layer, concentrate to 2 L and cool down to 0°C to precipitate a large amount of solid, remove it by filtration, and re- Concentrate to 1 L, and after cooling down, solids are precipitated again, filtered, and the solids obtained by two filtrations are combined, washed with a solution of PE / EA=5:1, filtered, and the solids (about 500 g) are removed, and the combined filtrates are decompressed to remove the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com