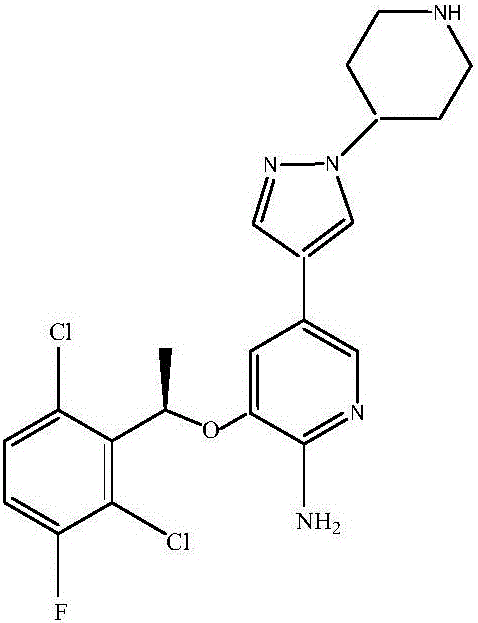
Synthetic method of crizotinib intermediate
A technology of crizotinib and intermediates, applied in the field of small molecule chemical drug preparation, can solve the problems of many reaction steps, low preparation cost, low intermediate yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
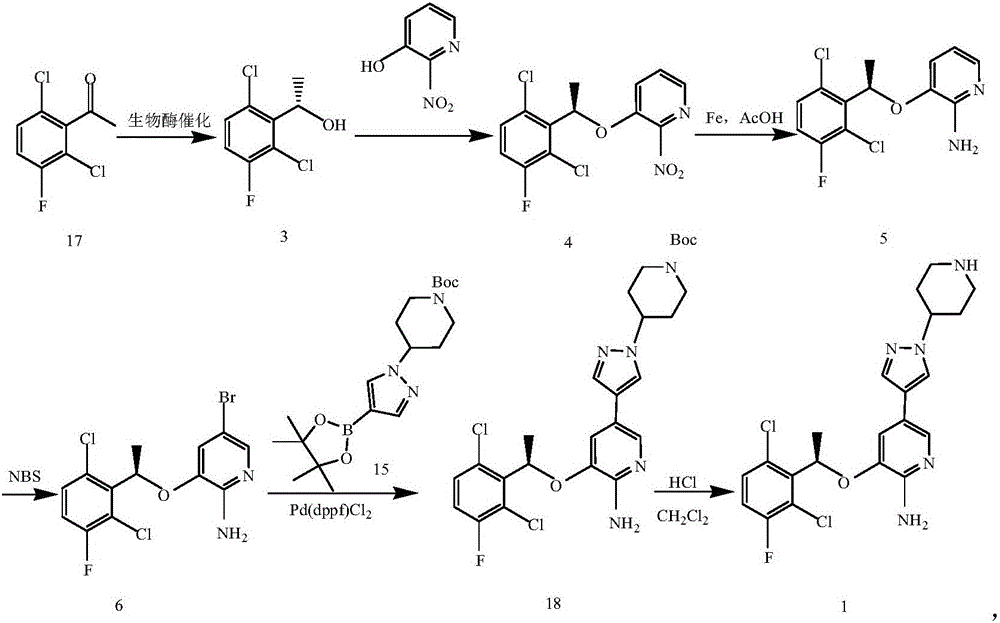
[0097] Example 1 Synthesis of S-1-(2,6-dichloro-3-fluorophenyl)ethanol
[0098]
[0099] Add purified water (828mL) and 2,6-dichloro-5-fluoro-acetophenone (1.0mol) into the reaction flask, stir well and add ketone reductase solution (9136mL), ammonium formate (2.0mol), NAD+ (0.03mol), adjust the pH of the system to 6.2-6.4, then raise the temperature of the system to 27-33°C and keep it warm for 17-24h. After the reaction, raise the temperature of the system to 65-70°C to destroy the enzyme protein, add ethyl acetate, pass through a silica gel pad, separate the liquids, back-extract the aqueous phase with ethyl acetate, combine the organic phases, and concentrate to obtain the product S-1-(2 , 6-dichloro-3-fluorophenyl) ethanol, the purity is 93-98%, the reaction yield is 85-95%, and the enantiomeric excess percentage (%e.e.) value is greater than 98%.
[0100] 1 H NMR (400MHz, CDCl3): δ: 7.32(d, J=7.8Hz, 1H), 7.04(d, J=8.0Hz, 1H), 4.89(q, J=7.2Hz, 1H), 1.48(d, J=7.6Hz, ...
Embodiment 2
[0101] Example 2 Synthesis of R-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-pyridin-2-amine (intermediate II)
[0102]
[0103] Reference method (Zhang Guangyan, Li Pengcheng, Liu Difa, et al. Research on the synthesis process of crizotinib. Chinese Journal of Medicinal Chemistry, 2014, 24(6): 445-449), making S-1-(2,6 -Dichloro-3-fluorophenyl)ethanol reacts with 2-nitro-3-hydroxypyridine to generate R-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2 -nitropyridine, its nitro group is reduced to obtain R-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-amine, and then the aromatic amine compound is brominated Substitution affords R-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-pyridin-2-amine.
Embodiment 3
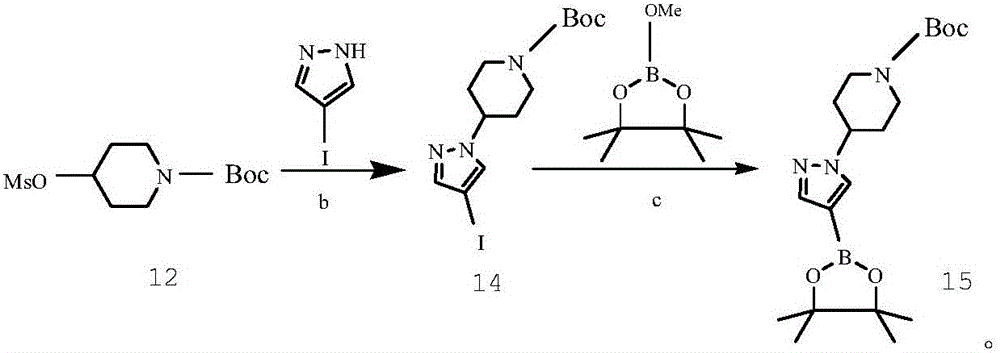
[0104] Example 3 Synthesis of 4-(4-bromo-1H-pyrazolyl)piperidine-1-carboxylic acid tert-butyl ester (compound 3)
[0105]
[0106] A reactor with specifications of 4*6mm was used to carry out 400g 4-mesylate piperidine-1-carboxylate tert-butyl ester (compound 1) in tetrahydrofuran (4000mL), 250.8g 4-bromopyrazole (compound 2) and tert-butyl Potassium alkoxide (240.9g tetrahydrofuran solution (6000mL) continuous feeding nucleophilic substitution reaction, coil length 5 ~ 8m, reaction temperature 50 ~ 60 ℃, reaction residence time 1 ~ 10min, continuous feeding reaction yield 80 ~ 90% After the continuous reaction system flows out of the coil, it is directly added to purified water to terminate the reaction. After the reaction is completed, the aqueous phase is directly extracted with ethyl acetate, and concentrated to obtain the product 4-(4-bromo-1H-pyrazolyl)piperidine-1- Tert-butyl formate (compound 3), with a purity of 93.2%, can be directly used for the next reaction.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com