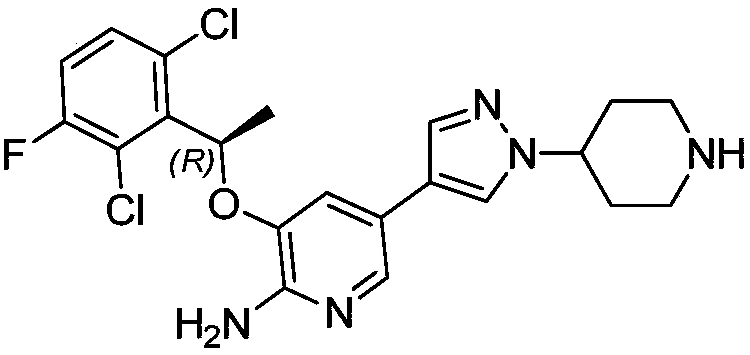
Palladium removal method for crizotinib intermediate
A technology for crizotinib and intermediates, which is applied in the field of palladium removal for crizotinib intermediates, can solve problems such as not being suitable for industrial production, and achieve the effects of meeting industrial production requirements, saving organic solvents, and being simple and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
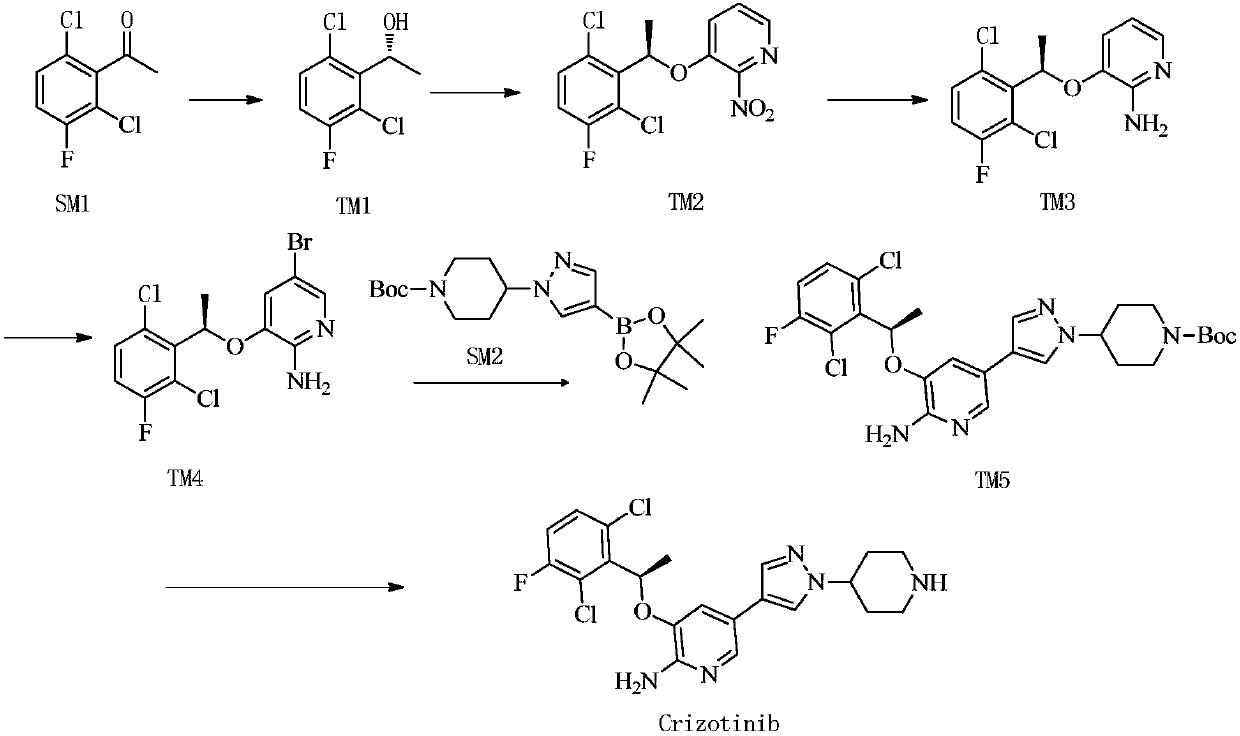
[0032] 1kg toluene, 238g (R)-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-amine (TM4) and 4-(4- (4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert Butyl ester (SM2), 1.5g tetrabutylammonium bromide, 240g cesium carbonate and 3.8g Pd(dppf)Cl 2 Add to the reaction bottle in turn, start stirring and add 1200g of purified water, replace with nitrogen for 3 times before adding, heat to 80-90°C under the protection of nitrogen, and keep stirring at 65-95°C for 3-6 hours.
[0033] After the reaction was completed, the temperature was lowered after the reaction was completed, the liquids were separated, the organic phase was washed once with 1000 L of water, and the aqueous phase was discarded.
[0034] Add 42.8g of N-acetyl-L-cysteine to the organic phase, stir at 50-70°C for 10-16h, add 1000L of potassium carbonate aqueous solution, wash, separate the liquid, and discard the aqueous phase; then use 1000L of potassium carb...
Embodiment 2
[0039] 1kg toluene, 238g (R)-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-amine (TM4) and 4-(4- (4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert Butyl ester (SM2), 1.5g tetrabutylammonium bromide, 240g cesium carbonate and 3.8g Pd(dppf)Cl 2 Add to the reaction bottle in turn, start stirring and add 1200g of purified water, replace with nitrogen for 3 times before adding, heat to 80-90°C under nitrogen protection, and keep stirring at 650-95°C for 3-6 hours.
[0040] After the reaction was completed, the temperature was lowered after the reaction was completed, the liquids were separated, the organic phase was washed once with 1000 L of water, and the aqueous phase was discarded.
[0041]Add 60 g of 1.3.5-triazine-2.4.6-trithiol trisodium salt to the organic phase, stir at 50-70°C for 3-10 hours, filter with suction, and discard the filter cake.
[0042] Heat the organic phase to 50-80°C, add 2000g of n-heptane, coo...
Embodiment 3
[0046] 1kg toluene, 238g (R)-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-amine (TM4) and 4-(4- (4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert Butyl ester (SM2), 1.5g tetrabutylammonium bromide, 240g cesium carbonate and 3.8g Pd(dppf)Cl 2 Add to the reaction bottle in turn, start stirring and add 1200g of purified water, replace with nitrogen for 3 times before adding, heat to 80-90°C under nitrogen protection, and keep stirring at 650-95°C for 3-6 hours.
[0047] After the reaction was completed, the temperature was lowered after the reaction was completed, the liquids were separated, the organic phase was washed once with 1000 L of water, and the aqueous phase was discarded.
[0048] Add 60g of mercapto silica gel to the organic phase, stir at 50-70°C for 3-10h, filter with suction, and discard the filter cake.
[0049] Heat the organic phase to 50-80°C, add 2000g of n-heptane, cool down to 5-25°C after additio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com