Method for refining histone deacetylase (HDAC) inhibitor vorinostat
A technology of vorinostat and refining method, which is applied in the field of refining vorinostat, a protein deacetylase inhibitor, can solve the problems of high production cost, unfavorable environmental protection requirements, unsuitability, etc., and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
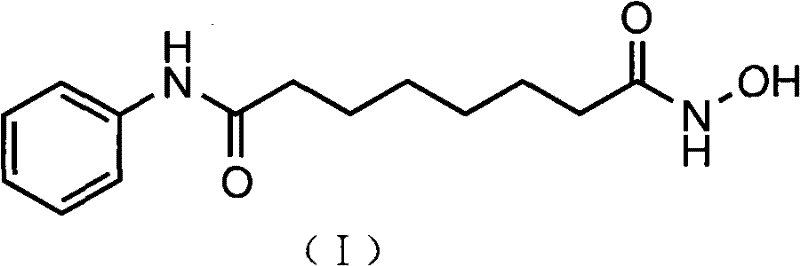
[0026] Synthesis of Vorinostat
[0027] 1. Synthesis of suberic anhydride:
[0028] ①Reaction formula:
[0029]
[0030] ②Operation method:
[0031] Add 1280 grams of suberic acid and 3.2 liters of acetic anhydride into a 10L reaction flask, heat and reflux for 1.5 hours under the protection of nitrogen, evaporate the solvent under vacuum, add 3.2 liters of acetonitrile to dissolve, and stand at -15°C overnight, and a large amount of white solids are precipitated , filtered, the solid was washed with 1.9 liters of acetonitrile at -15°C, and dried by suction, and the filter cake was vacuum-dried at room temperature to a constant weight to obtain 1100 grams of white powder.
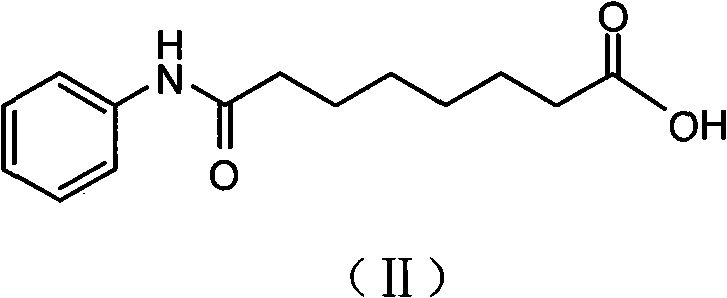
[0032] 2. Synthesis of Octanoanilic Acid:
[0033] ①Reaction formula:
[0034]
[0035] ②Operation method:
[0036] At room temperature, add 720 grams of suberic anhydride and 6350 ml of tetrahydrofuran into a 10L reaction bottle, stir to dissolve, add 432 grams of aniline dropwise, and then stir...
Embodiment 2
[0043] Water (sodium bicarbonate) crystallization method of vorinostat
[0044] In Example 1, 436 grams of crude product (without drying, containing about 43% moisture) was refluxed with 43.6 liters of water to dissolve, and then 250ml of aqueous sodium bicarbonate solution (10 g / liter) was added to cool down to below 30°C to precipitate orange-yellow powder, which was filtered , dried to obtain 225 grams of orange powder (HPLC normalization method detected caprylanilic acid containing about 0.7%).
Embodiment 3
[0046] Water crystallization method of vorinostat
[0047] 6.75 liters of water was heated to reflux, 25 grams of the product of Example 2 was added, and stirred to dissolve. Cool down to below 30°C to precipitate an orange powder, filter, and dry to obtain 22 grams of an orange powder (octanoanilide acid content is about 0.3% detected by HPLC normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com