New methods and pure polymorphs
A technology of polymorphs and crystal forms, applied in the field of new and pure polymorphs, can solve problems such as difficult reproduction, impure polymorphs, and inconsistencies, and achieve extremely high repeatability and convenient repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
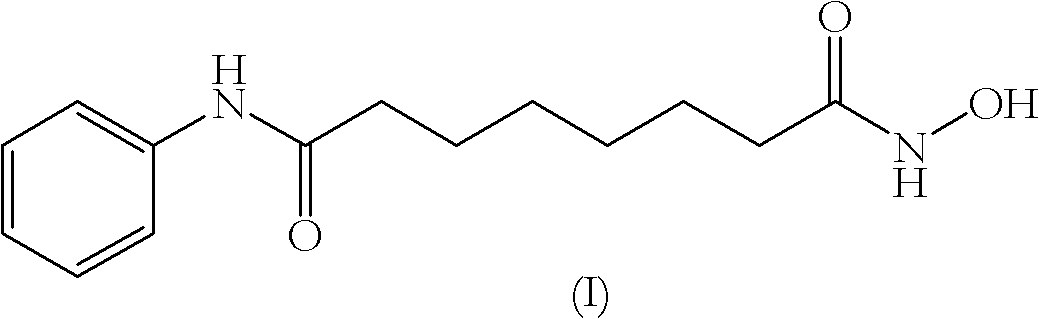
[0068] Embodiment 1: Preparation of vorinostat crystal form I
[0069] Vorinostat (10 g) was charged into a reaction flask containing amide (50 ml) (organic solvent). The resulting suspension was heated at 60° C. for 1 hour with stirring. The resulting clear solution was poured into water (250ml) at 20°C to 25°C. A white solid precipitated out. The solid product was filtered and dried in vacuo at 60°C until constant weight was obtained.
[0070] Amide used
Embodiment 2
[0071] Embodiment 2: Preparation of vorinostat crystal form I
[0072] Vorinostat (10 g) was charged into a reaction flask containing alcohol (50 ml) (organic solvent). The resulting suspension was heated at 60° C. for 1 hour with stirring. The resulting clear solution was poured into water (50ml) at 20°C to 25°C. A white solid precipitated out. The solid product was filtered and dried in vacuo at 60°C until constant weight was obtained.
[0073] Alcohol used
Embodiment 3
[0074] Embodiment 3: Preparation of vorinostat crystal form I
[0075] Vorinostat (10 g) was charged into a reaction flask containing methanol (50 ml) (organic solvent). The suspension was heated at 60° C. for 1 hour with stirring. The resulting clear solution is poured into water (50-300ml, typically 50ml when the organic solvent is alcohol, typically 250ml when the organic solvent is an amide). The reaction mixture was cooled to 25°C and filtered. The obtained solid product was dried in vacuo at 60° C. until a constant weight was obtained.
[0076] Chemical purity ≥99.9% (as measured by HPLC)
[0077] Use different organic solvents to repeat the above-mentioned procedure (step) in embodiment 3 to obtain vorinostat crystal form I, namely:
[0078] Organic solvents: methanol, ethanol, isopropanol, 1-butanol, 2-butanol, tert-butanol, N,N-dimethylformamide, N,N-dimethylacetamide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com