Application of vorinostat in oxidation responded cationic polymer gene vector mediated gene therapy
A cationic polymer, gene carrier technology, applied in the field of biomedicine, to enhance the effect of gene transfection, improve the therapeutic effect, and promote the effect of charge reversal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
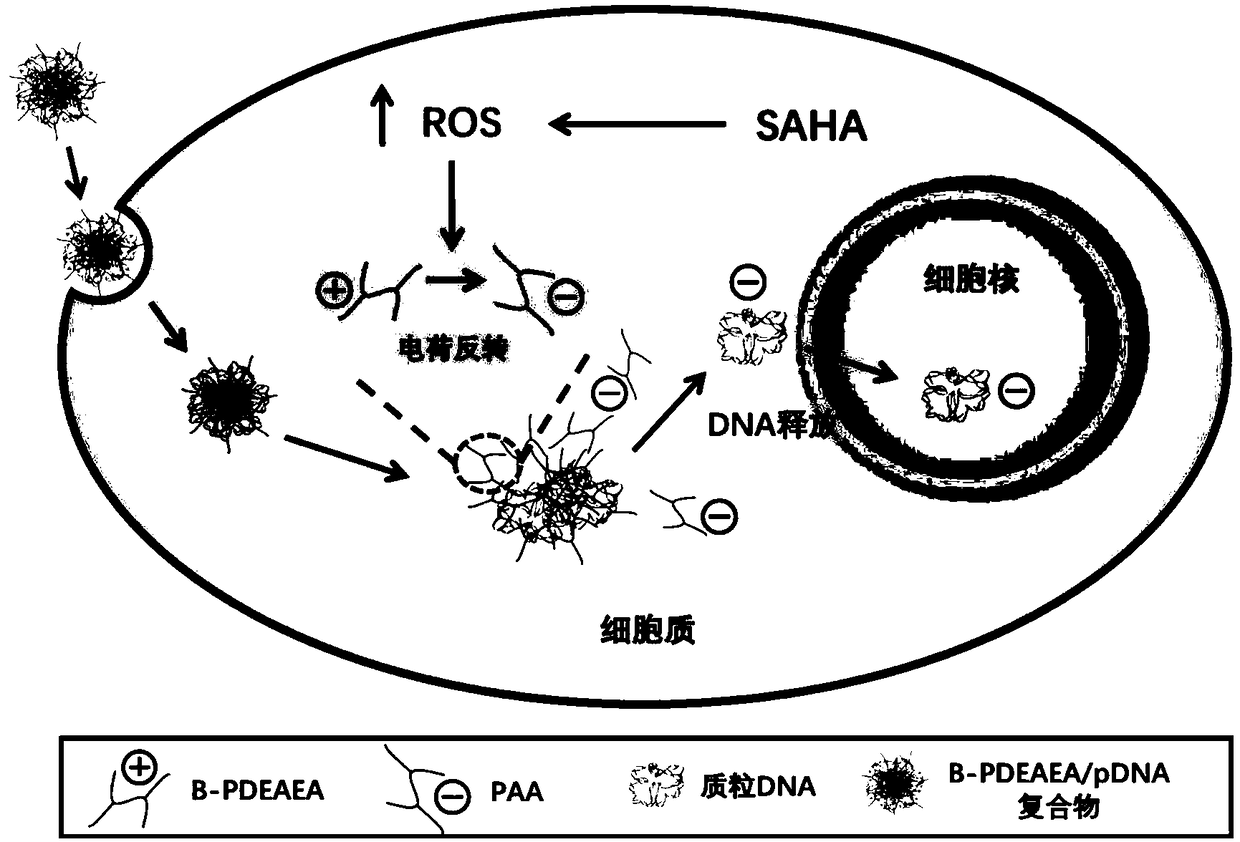
[0044] The cultivation of embodiment 1 cell and the preparation of nanocomposite
[0045] A549 cells and HeLa cells were cultured in RPMI-1640 medium containing 10% FBS in a constant temperature incubator, and the temperature of the incubator was set at 37°C, CO 2 The concentration is 5%.
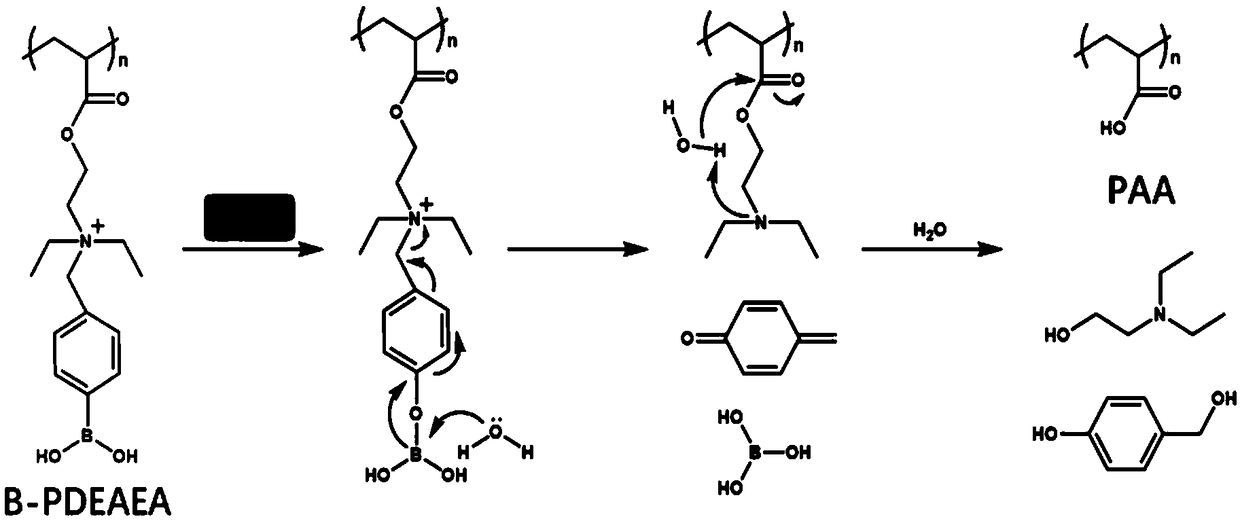
[0046]B-PDEAEA / DNA complex configuration: Dilute DNA to 40 μg / mL with pH 7.4 HEPES buffer, weigh an appropriate amount of B-PDEAEA and dissolve it with HEPES to dilute to 507 μg / mL, mix the two in equal volume and vortex for 10 seconds , standing for 30min, that is to form nanoparticles (polyplex, B-PDEAEA / DNA complex).
Embodiment 2
[0047] Example 2 Intracellular reactive oxygen species (ROS) detection
[0048] By using 2,7-dichlorofluorescein diacetate to detect the level of ROS in the cell, 2,7-dichlorofluorescein diacetate can be converted into a fluorescent substance dichlorofluorescence under the action of ROS after entering the cell yellow.
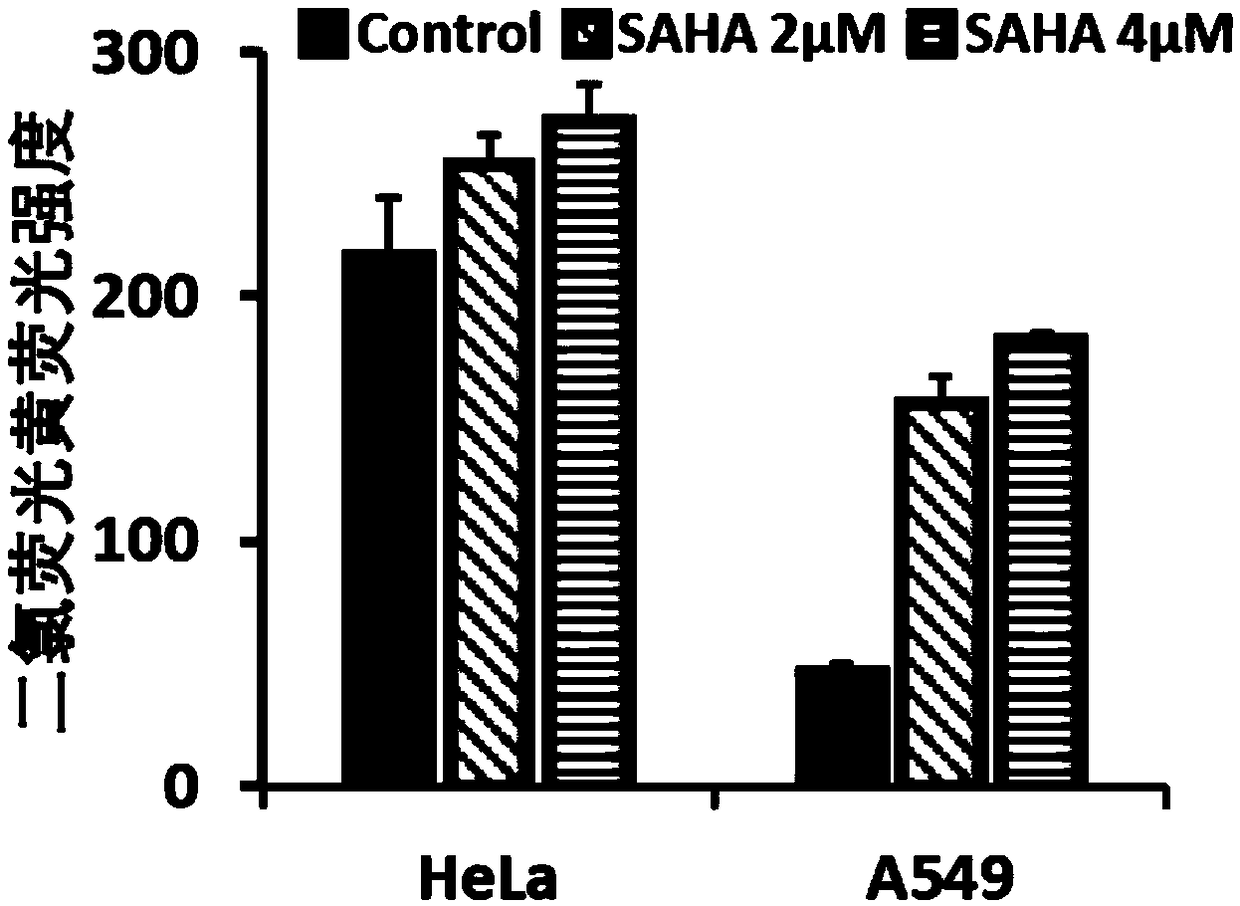
[0049] HeLa and A549 cells were plated in 6-well plates, and the number of cells was 200,000 / well; after the cells adhered to the wall, different concentrations of vorinostat were added to the experimental group (the concentration of SAHA was 2 μM or 4 μM), and no vorinostat was added to the control group; Cultivate for 24 hours, discard the medium, add 1ml of 2,7-dichlorofluorescein diacetate at a concentration of 10μM and incubate at 37°C for 30 minutes, collect the cells and detect the fluorescence intensity of dichlorofluorescein by flow cytometry; excitation wavelength 488nm, emission wavelength 520nm.
[0050] The results showed that after A549 cells we...
Embodiment 3
[0051] Example 3 Cytotoxicity Detection
[0052] 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (MTT) method was used to detect the effect of transfection of TRAIL plasmid on the growth of PSCs.
[0053] Detection principle: Mitochondria of living cells can produce succinate dehydrogenase, under the action of this enzyme, MTT will be reduced to water-insoluble blue-purple crystalline formazan (Formazan) to form deposits, dead cells will not cause this reaction . Dimethyl sulfoxide (DMSO) can dissolve the generated formazan crystals, measure its maximum light absorption value at a wavelength of 562nm with a microplate reader, and subtract the background absorption value at a wavelength of 620nm, thereby indirectly reflecting the cell growth in the experiment.
[0054] 200 μL containing 4000 A549 or HeLa cell suspensions were added to each well of a 96-well plate, and then the cells were placed in a 37° C. constant temperature incubator (5% CO2 concentration, 95% hu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com