Application of vorinostat to preparation of lung cancer treatment drug
A technology of vorinostat and drugs, applied in the field of biopharmaceuticals, can solve the problems of lung cancer safety and effectiveness, adverse reactions of lung cancer, difficult popularization and application, etc., achieve good development prospects, inhibit tumor cell growth, and facilitate supervision Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]Embodiment 1 The preparation of vorinostat solution
[0028] Vorinostat was purchased from Sigma-aldrich Company, CAS registration number: 329825127.
[0029] DMSO (dimethyl sulfoxide) was purchased from Singma-aldrich Company.
[0030] Vorinostat was prepared into a 100mM solution with dimethyl sulfoxide, and diluted to the required concentration with PBS (phosphate buffer saline) before use in cell experiments; in animal experiments, directly weigh 0.04g of vorinostat solution Prepare a 40mg / mL solution in 1mLDMSO, then take 100µl of the above 40mg / mL vorinostat solution and dilute it with 10mL of PBS to prepare a 4mg / mL vorinostat solution for use.
Embodiment 2
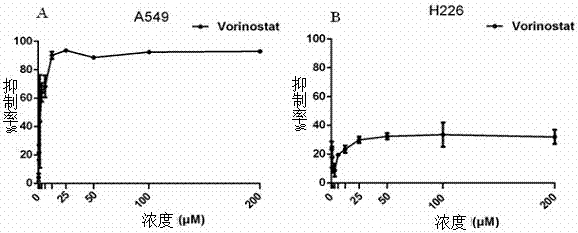
[0031] Example 2 The inhibitory effect of vorinostat on the proliferation of A549 and H226 cells
[0032] The human non-small cell lung cancer cell line A549 (purchased from the China Atypical Collection Center) and the human lung squamous cell carcinoma cell line H226 (purchased from the China Atypical Collection Center) in the logarithmic growth phase were used at 1000-2000 The density of cells / well was seeded in a 96-well plate, and after 24 hours, it was replaced with different concentrations of ) Vorinostat’s complete medium was used to treat the cells for 72 hours, and the cell proliferation was detected by the CCK-8 method (cell activity toxicity detection method). For the detection method, see "Medical Immunology Experimental Technology" (Soochow University Press, publication date: February 2011).
[0033] Such as figure 1 As shown, as the concentration increases, the IC50 (half maximal inhibitory concentration, half inhibitory concentration) value of vorinostat inhi...
Embodiment 3
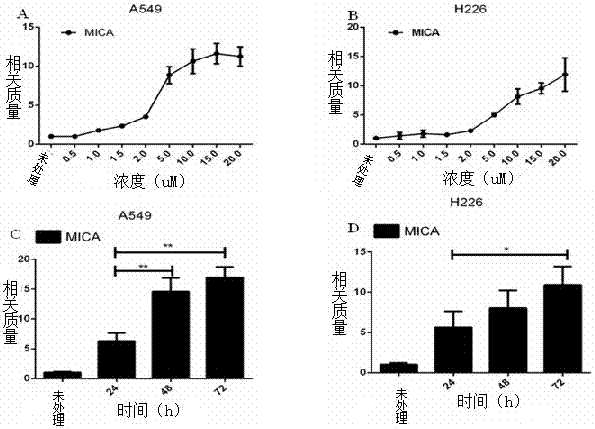
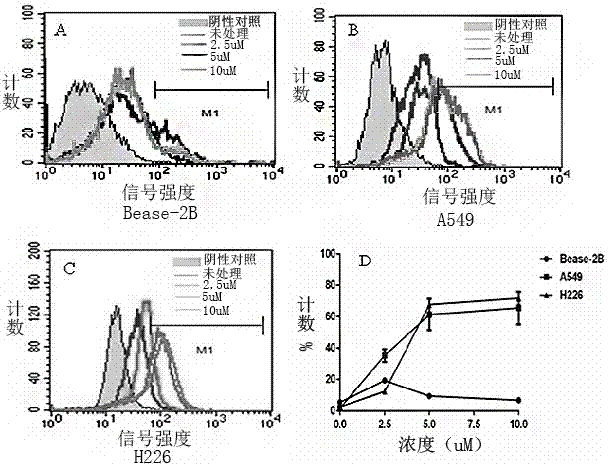
[0034] Example 3 Vorinostat promotes the expression of MICA mRNA in A549 and H226 cells
[0035] 1. Trizol method to extract cellular RNA:
[0036] (1) A549 and H226 cells in the logarithmic growth phase (purchased from China Atypical Collection Center) were treated with concentrations of 0 μM, 0.5 μM, 1.0 μM, 1.5 μM, 2.0 μM, 5 μM, 10 μM, 15 μM, 20 μM vorinostat solution for 72h and 10 μM vorinostat for 0h, 24h, 48h, and 72h;
[0037] (2) Then wash the above-mentioned A549 and H226 cells treated with vorinostat solution twice with PBS (centrifuge at 2000rpm for 5min), then add 1mL Trizol reagent, shake slightly, and pipette evenly to ensure that the lysate is evenly distributed on the cell surface;
[0038] (3) Transfer the above-mentioned lysate containing cells to a 1.5mL EP tube (centrifuge tube), pipette repeatedly with a pipette until the lysate is fully lysed without obvious precipitation, and then stand at room temperature for 5 minutes;
[0039] (4) Add 200 μL of chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com