A c-met/hdac dual-target inhibitor based on the structure of crizotinib and its synthesis method and application
A technology of crizotinib and a synthesis method, which is applied in the field of medicine and achieves the effects of mild conditions, high yield and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
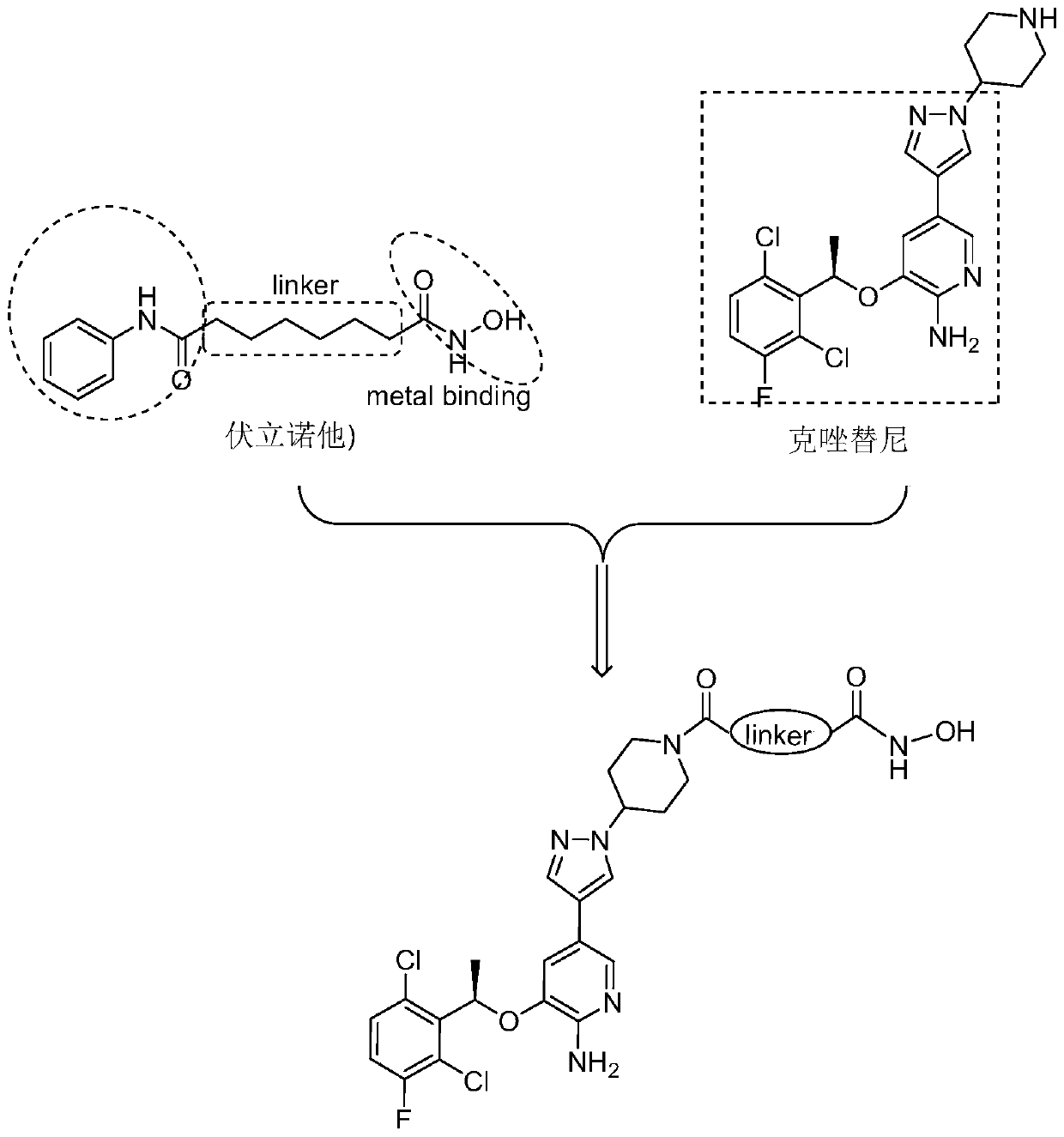
[0040] The structural formula of the c-Met / HDAC dual-target inhibitor based on the structure of crizotinib in this example is as follows:
[0041]
[0042] Among them, the linker is n=4.
[0043] The synthesis method of the dual-target inhibitor of this embodiment is as follows:
[0044] (1) at first synthetic intermediate (a) reaction equation is as follows:
[0045]
[0046] Dissolve monomethyl adipate (320.3mg, 2.0mmol) in 30ml DMF, then add HATU (912.6mg, 2.4mmol) and DIPEA (517.0mg, 4.0mmol), stir for 15min under ice bath conditions, then add Crizotinib (945.7mg, 2.1mmol) was stirred at room temperature for 4h, and TLC monitoring (volume ratio of dichloromethane to methanol: 10:1) showed that the reaction of the raw material was basically complete. Add 30ml of water and 80ml of ethyl acetate, separate the organic phase, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure. Column chromatography of the resi...
Embodiment 2
[0059] In the structural formula of the c-Met / HDAC dual-target inhibitor based on the structure of crizotinib in this example, the linker is n=5, synthesized according to the method of Example 1, monomethyl adipate was replaced by monomethyl pimelate, and the test parameters were routinely adjusted to obtain the target product (I-2). The 1HNMR data were as follows:
[0060] 1HNMR (400MHz, DMSO-d6):ppm: 10.39(s,1H,),8.78(s,1H),7.99(s,1H),7.76(d,J=1.68Hz,1H),7.54-7.59(m ,2H),7.45(t,J=8.68Hz,8.68Hz,1H),6.90(d,J=1.4Hz,1H),6.06-6.11(q,J=6.44Hz,6.72Hz,6.44Hz,1H) ,5.69(s,2H),4.46-4.49(d,J=12.36Hz,1H),4.36-4.42(m,1H),3.96(d,J=13.44Hz,1H),3.17(t,J=11.80 Hz,12.6Hz,1H),2.71(t,J=12.36Hz,11.2Hz,1H),2.33(t,J=7.6Hz,7.28Hz,2H),1.93-2.06(m,4H),1.69-1.86 (m,5H), 1.46-1.54(m,4H), 1.22-1.30(m,2H).
Embodiment 3
[0062] In the structural formula of the c-Met / HDAC dual-target inhibitor based on the structure of crizotinib in this example, the linker is n=6, synthesized according to the method of Example 1, monomethyl adipate was replaced by monomethyl suberate, and the test parameters were routinely adjusted to obtain the target product (I-3). The 1HNMR data were as follows:
[0063] 1H NMR (400MHz, DMSO-d6): ppm: 10.40(s, 1H), 8.78(s, 1H), 7.98(s, 1H), 7.75(d, J=1.52Hz, 1H), 7.54-7.59(m ,2H),7.45(t,J=6.86Hz,6.86Hz,1H),6.90(d,J=1.68Hz,1H),6.06-6.11(q,J=6.72Hz,6.76Hz,6.44Hz,1H) ,5.69(s,2H),4.47(d,J=12.6Hz,1H),4.35-4.43(m,1H),3.95(d,J=13.44Hz,1H),3.13-3.20(t,J=12.32 Hz, 2.04Hz, 1H), 2.68-2.74(t, J=11.36Hz, 11.92Hz, 1H), 2.31-2.35(t, J=7.56Hz, 7.28Hz, 2H), 1.92-2.06(m, 4H) ,1.68-1.86(m,5H),1.07-1.50(m,8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com