Pharmaceutical composition containing gefitinib and histone deacetylase inhibitor, lipidosome preparation of pharmaceutical composition, and pharmaceutical application of pharmaceutical composition and lipidosome preparation
A technology of deacetylase and gefitinib, applied in the field of biomedicine, can solve the problems of narrow therapeutic window, failure of molecular targeted therapy, and limited clinical benefit, and achieve the effect of improving accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
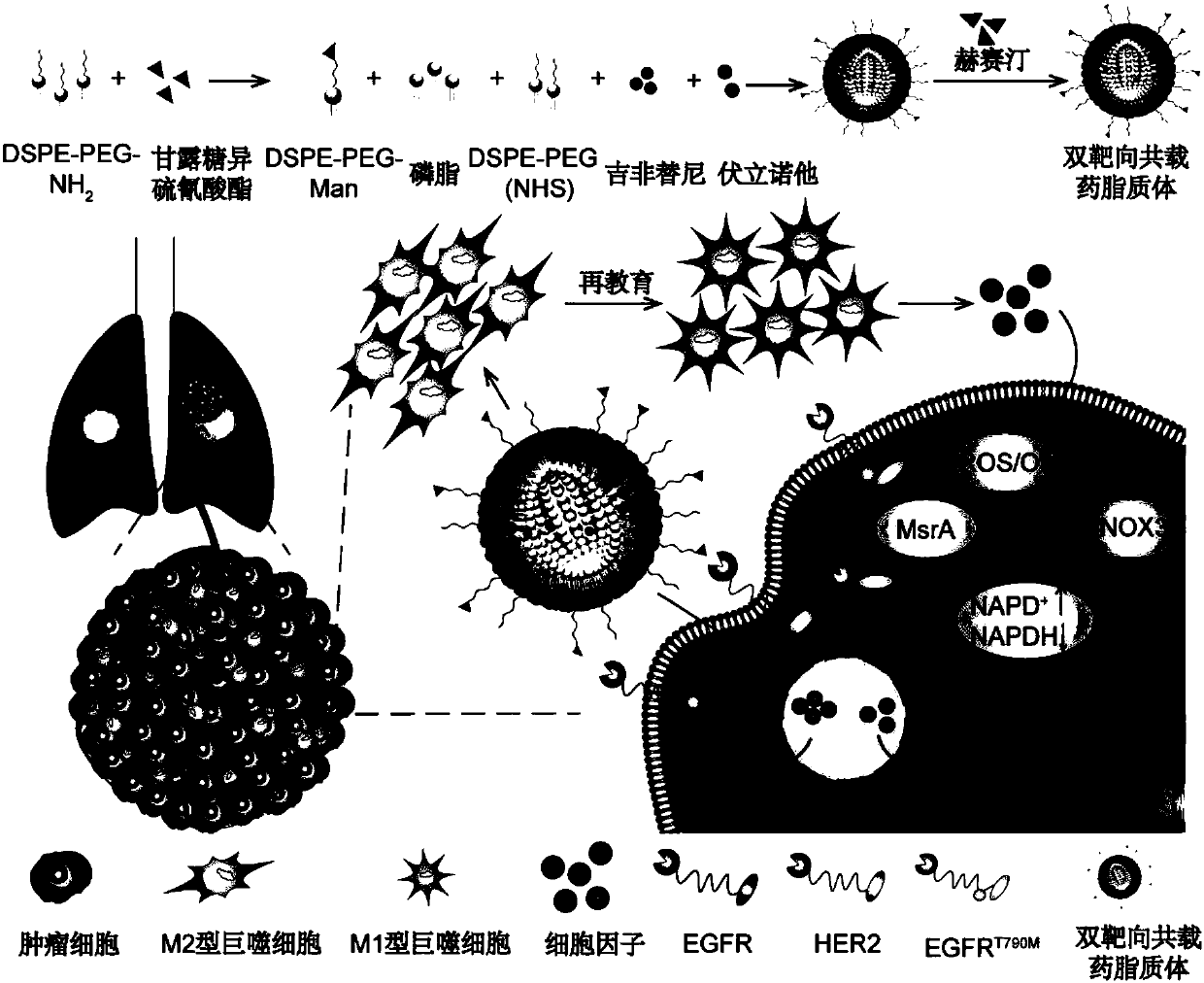
[0068] Preparation of dual-targeted co-loaded liposomes
[0069] (a) Weigh an appropriate amount of mannose isothiocyanate and dissolve it in dichloromethane, add an appropriate amount of triethylamine to adjust the pH to alkaline, and use mannose isothiocyanate / DSPE-PEG 2000 -NH 2 The molecular ratio is 3 / 1, and then add an appropriate amount of DSPE-PEG to it 2000 -NH 2 , react at room temperature for 4 hours, remove the organic solvent by rotary evaporation, add an appropriate amount of water, shake well, centrifuge at 15,000 rpm for 5 minutes, take the supernatant and freeze-dry to obtain mannose-modified DSPE-PEG 2000 , call it DSPE-PEG 2000 -Man.
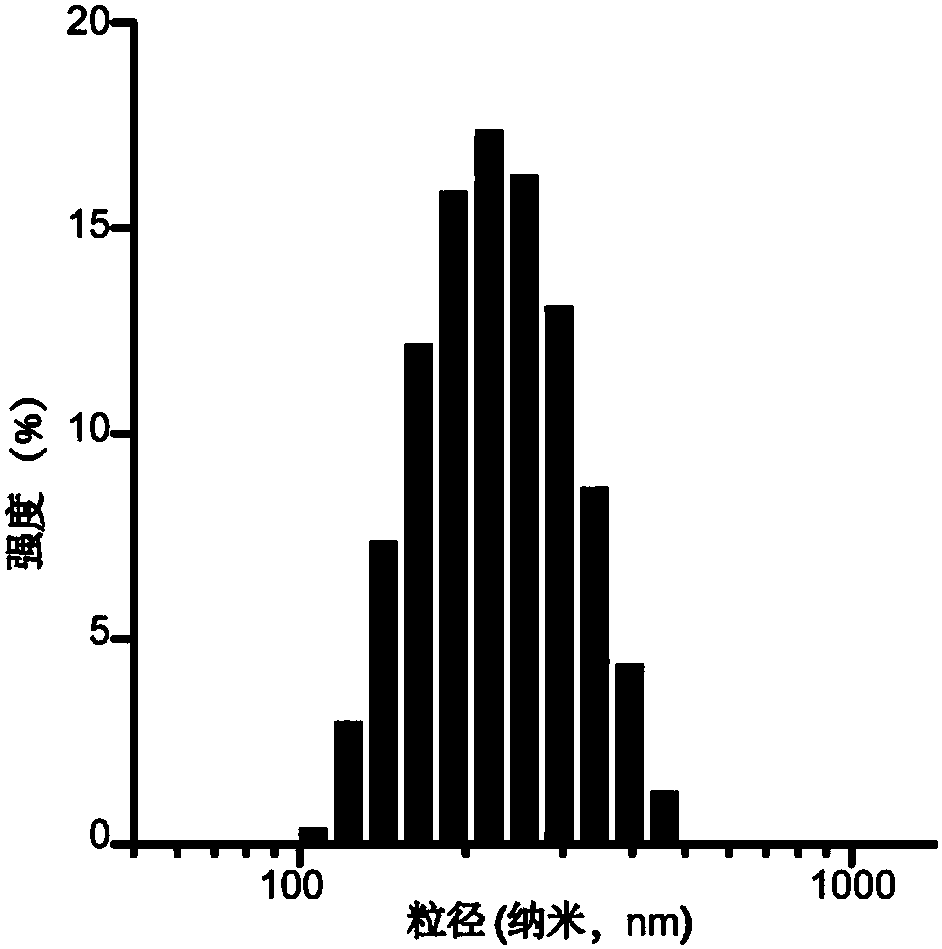
[0070] (b) Weigh egg yolk lecithin (PC 98T), cholesterol, DSPE-PEG 2000 、DSPE-PEG 2000 -Man and DSPE-PEG 2000 -NHS was dissolved in chloroform and added to the round bottom flask according to the molecular ratio of 10 / 2 / 1 / 0.1 / 0.1. Weigh gefitinib and vorinostat, dissolve them in methanol, add them into a round bottom f...
experiment Embodiment 1
[0090] in vitro cellular uptake
[0091] The green fluorescent probe coumarin 6 was introduced into liposomes at a mass ratio of gefitinib / coumarin 6 of 10 / 1 to prepare dual-targeted co-drug-loaded lipids loaded with coumarin 6 Plastids and unmodified drug-loaded liposomes, using a fluorescence spectrophotometer to quantify the coumarin 6 contained in the liposomes under the condition of an excitation wavelength of 466nm and an emission wavelength of 504nm, the amount of coumarin 6 entering the cell To characterize the cell entry efficiency of liposomes. details as follows:
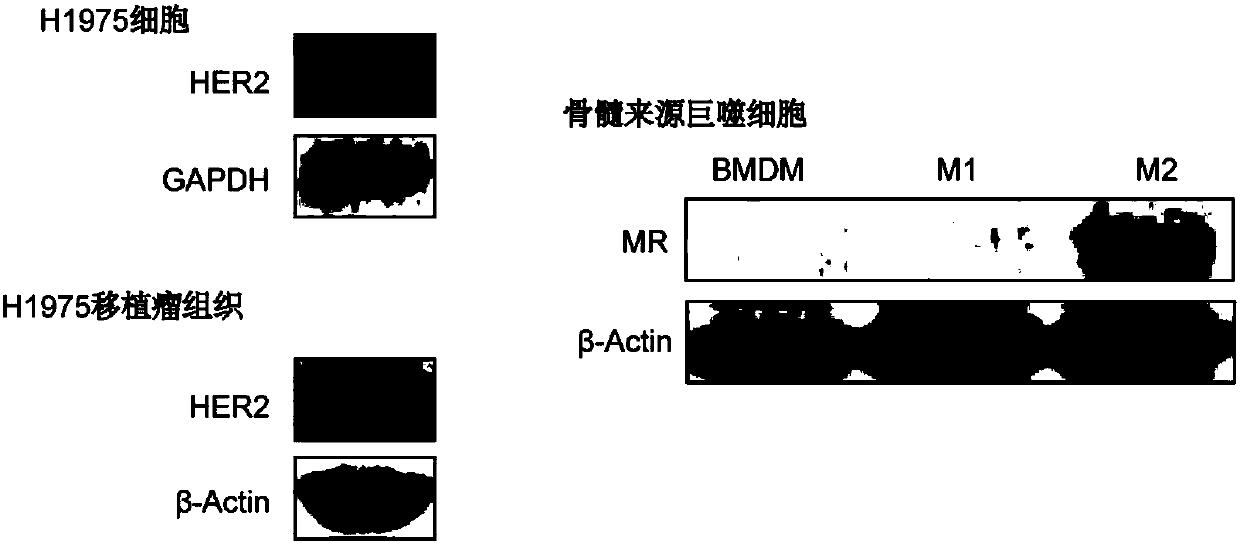
[0092] HER2-positive cell line H1975 and M2 macrophages were used at 8x10 per well 4 Cells were seeded in a 12-well cell culture plate at 37°C, 5% CO 2 After culturing under the same conditions for 24 hours, 0.1 μg of coumarin 6 per well was added to dual-targeted co-drug-loaded liposomes and unmodified co-drug-loaded liposomes. Cells were collected after liposome treatment for 4 hours for flow cytomet...
experiment Embodiment 2
[0095] In vitro antitumor activity
[0096] The in vitro toxicity of drugs and dual-targeted co-loaded liposomes in HER2-positive H1975 cells was detected by MTT method to characterize the impact of targeted modification and co-loading on the synergistic efficiency of combined drugs. The specific experiment is as follows:
[0097] at 5x10 3 Cells per well were planted in a 96-well plate at a density of 37°C, 5% CO 2After culturing for 24 hours under the same conditions, the cells were treated with different concentrations of gefitinib (the mass ratio of vorinostat to gefitinib was 0.12:1, and the mass ratio of TMP 195 to gefitinib was 1:1). Set up 6 auxiliary wells, and set up a negative control without drug addition at the same time. After culturing for 48 hours, 20 μL of 5 mg / mL MTT solution was added to each well, and cultured for another 4 hours. The liquid in each well was removed with a syringe, and 200 μL of dimethyl sulfoxide was added to each well. For the absorba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com