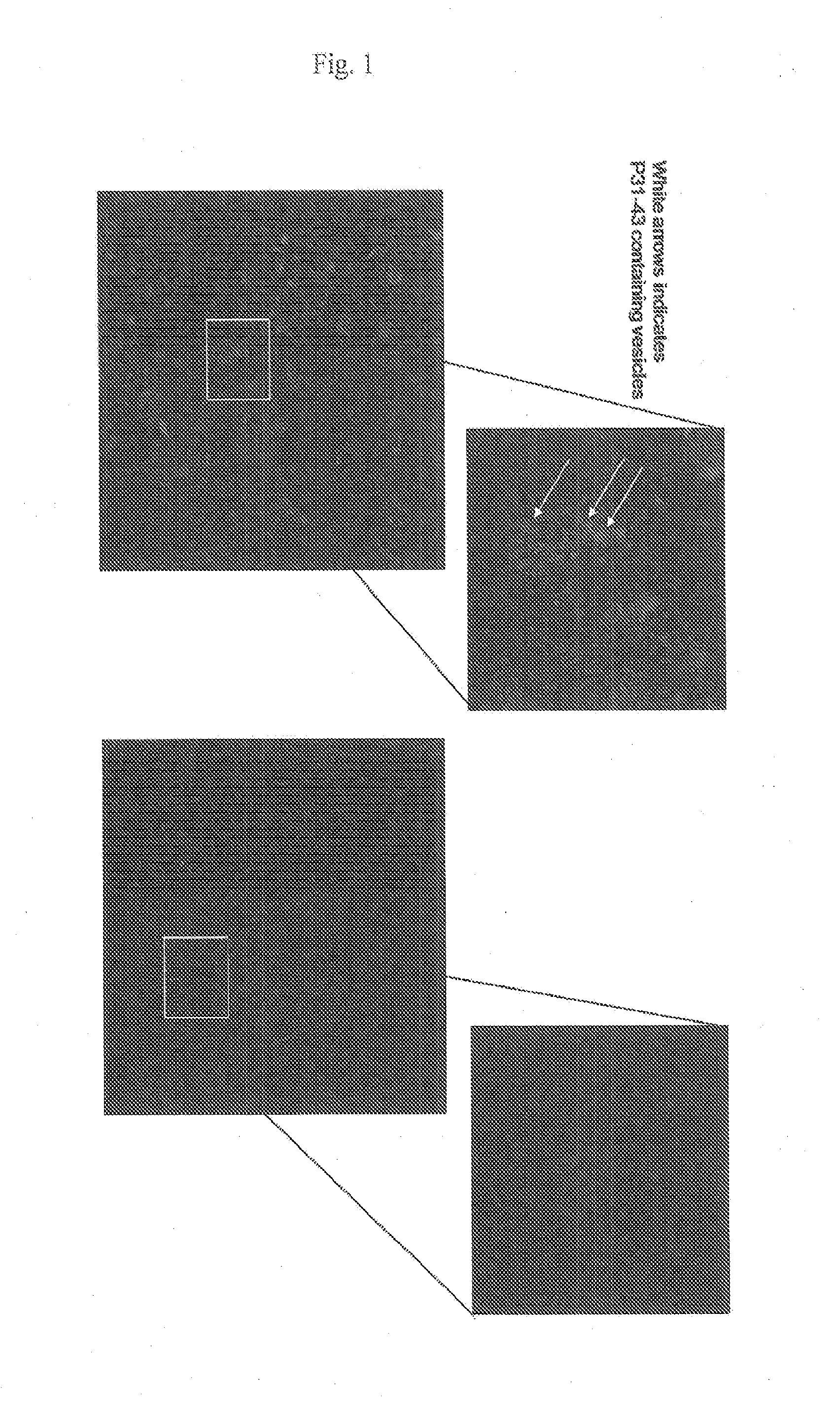
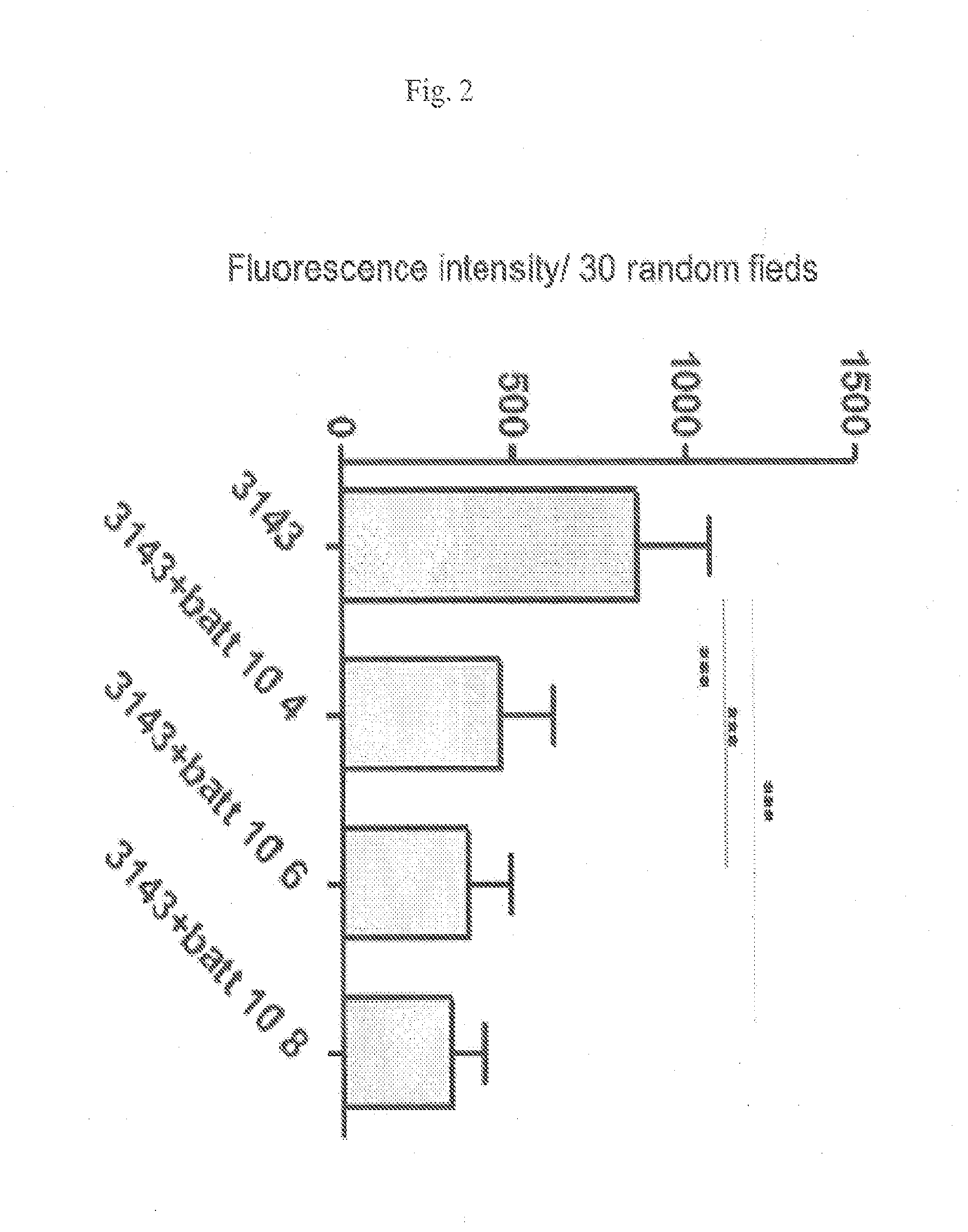
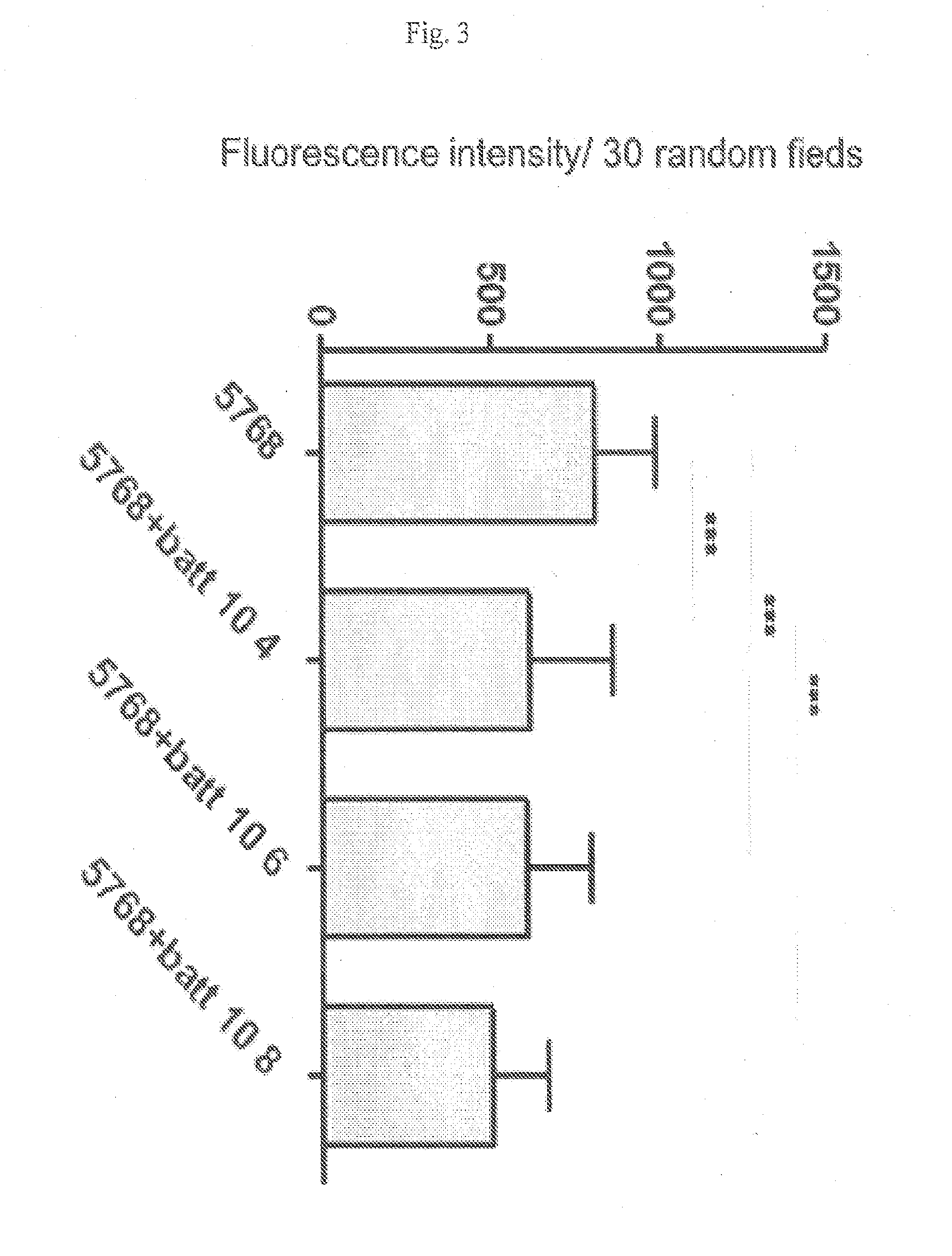
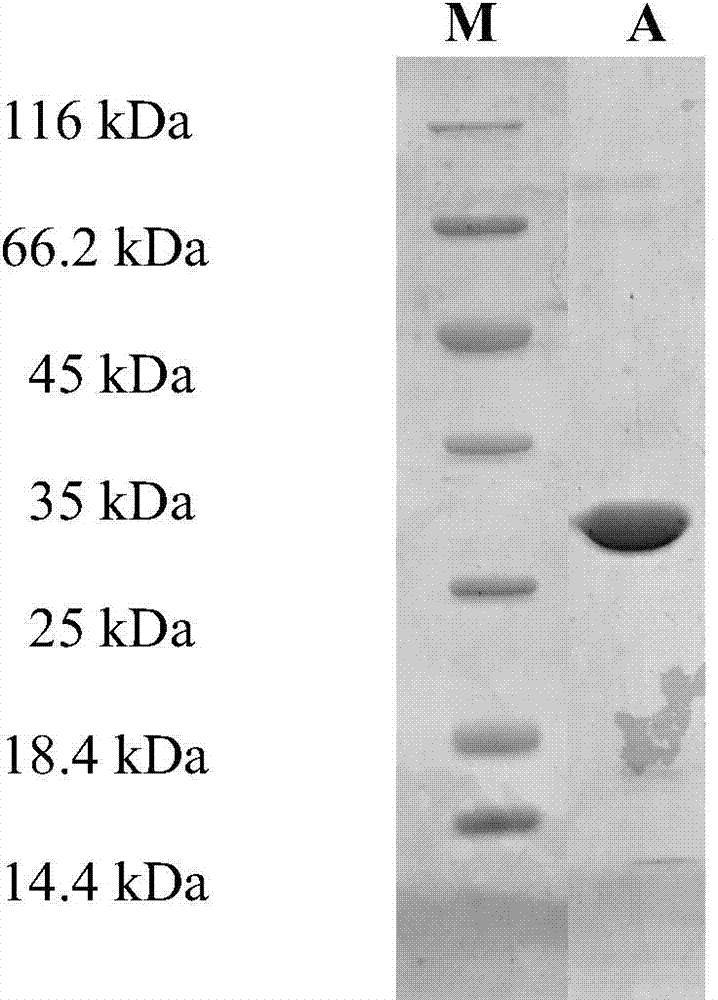
Patents
Literature
99 results about "Cell entry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
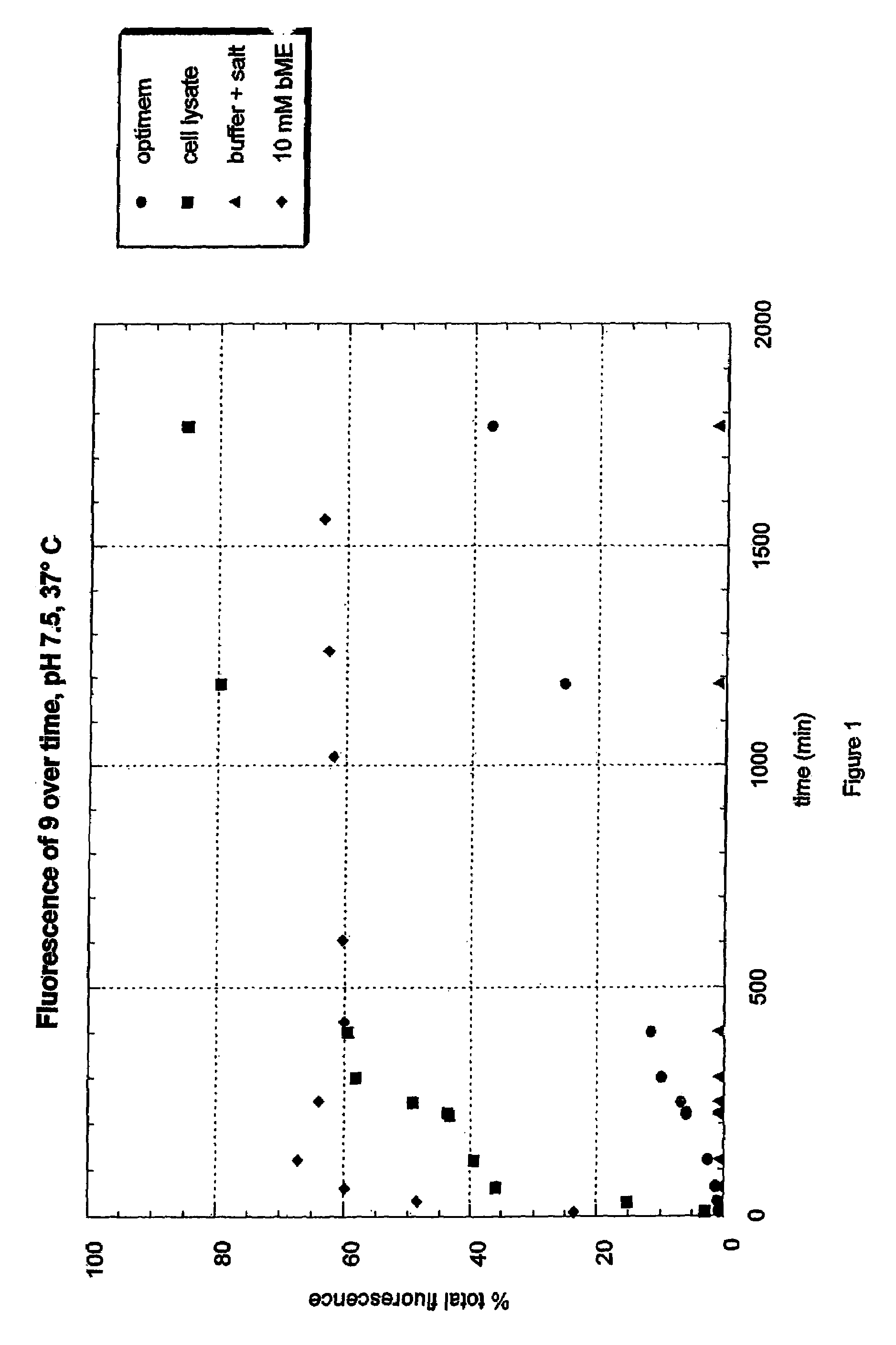
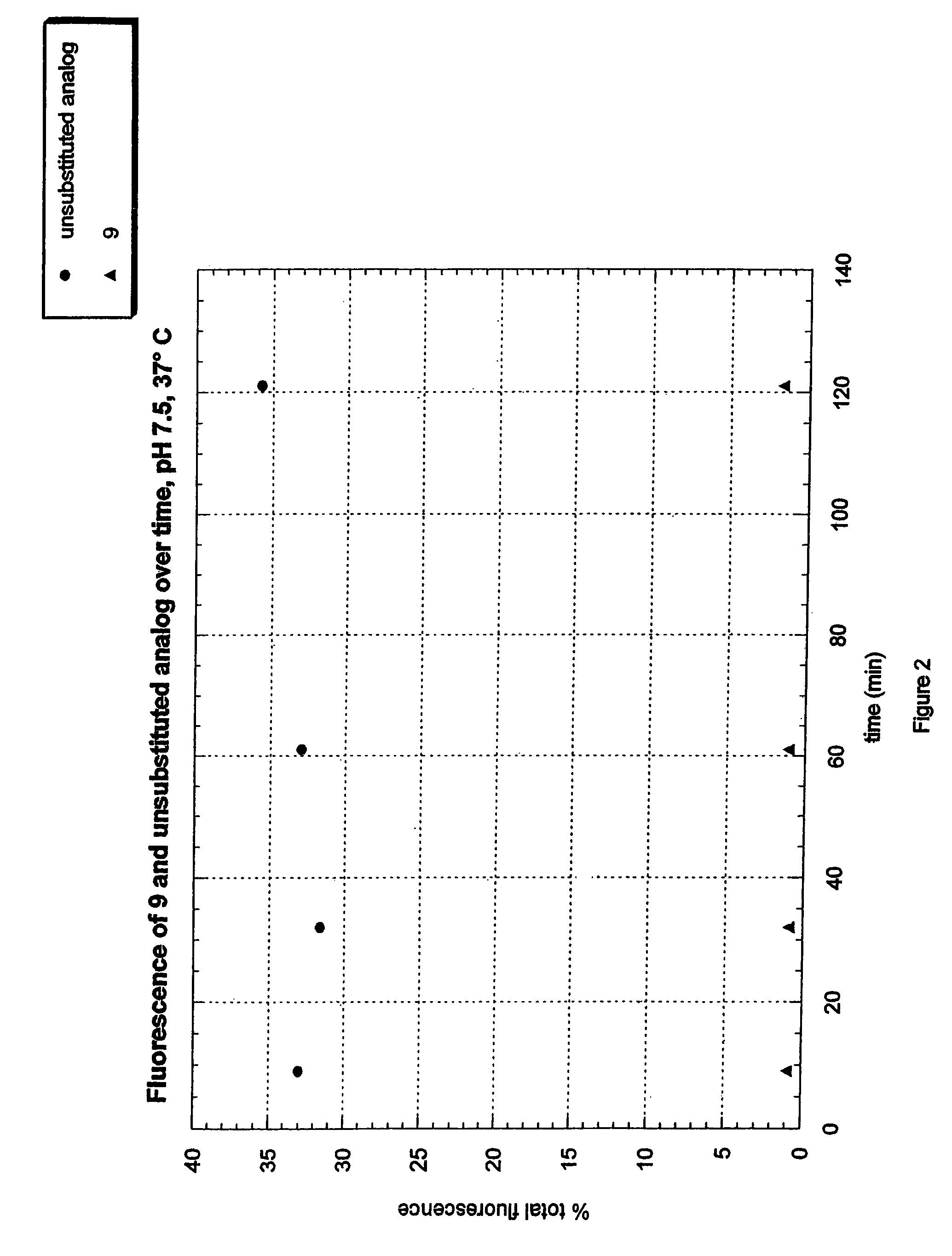
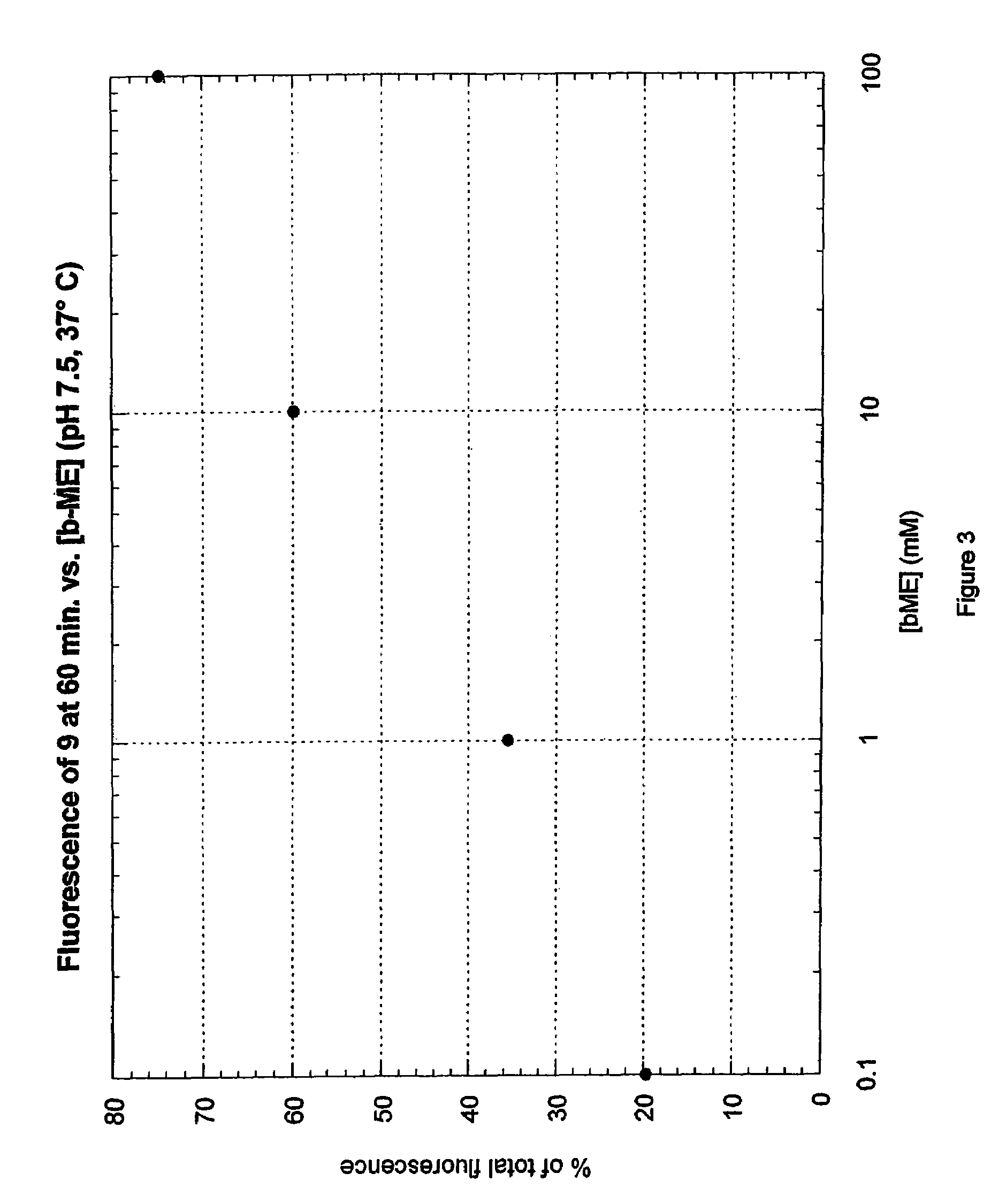
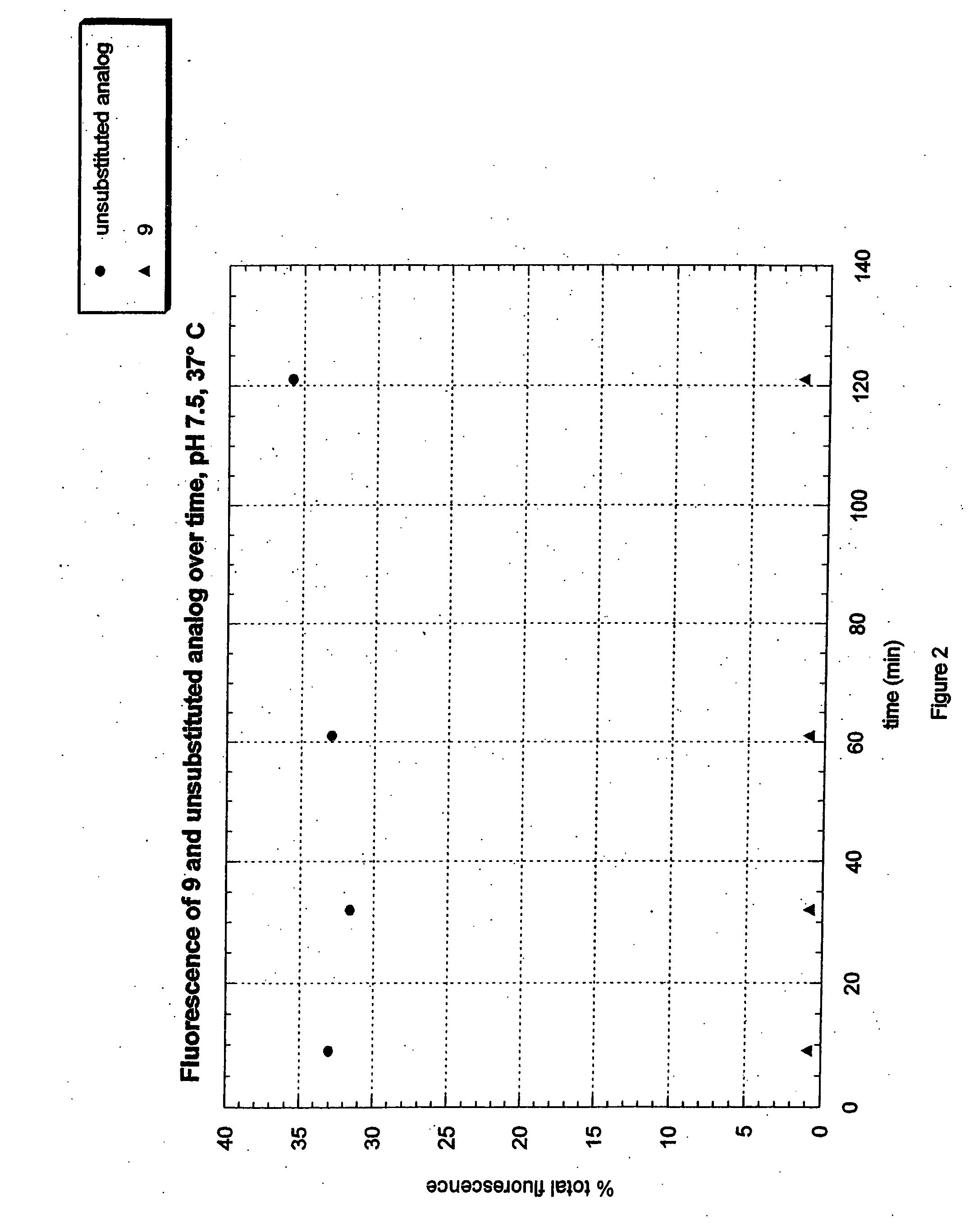
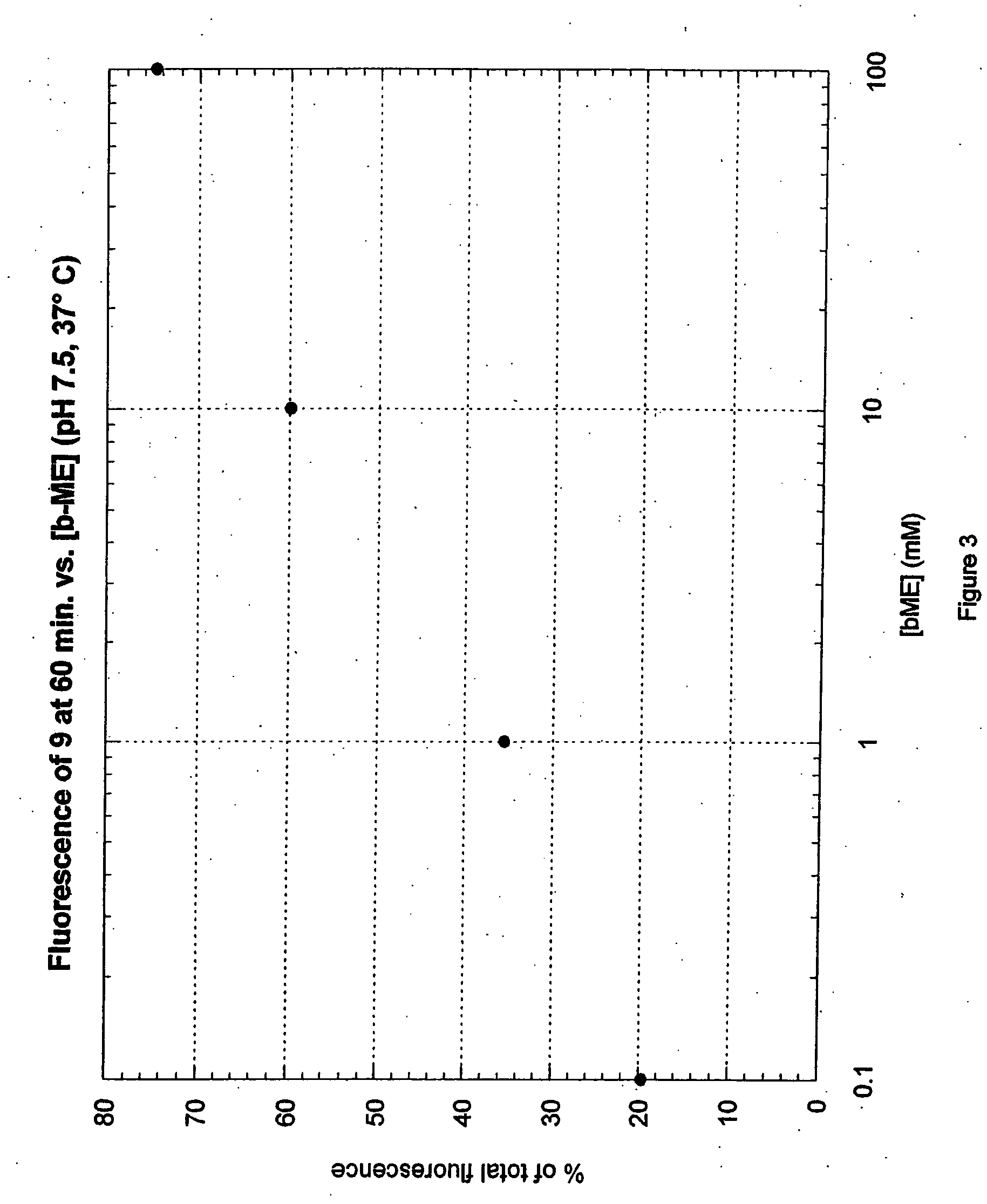
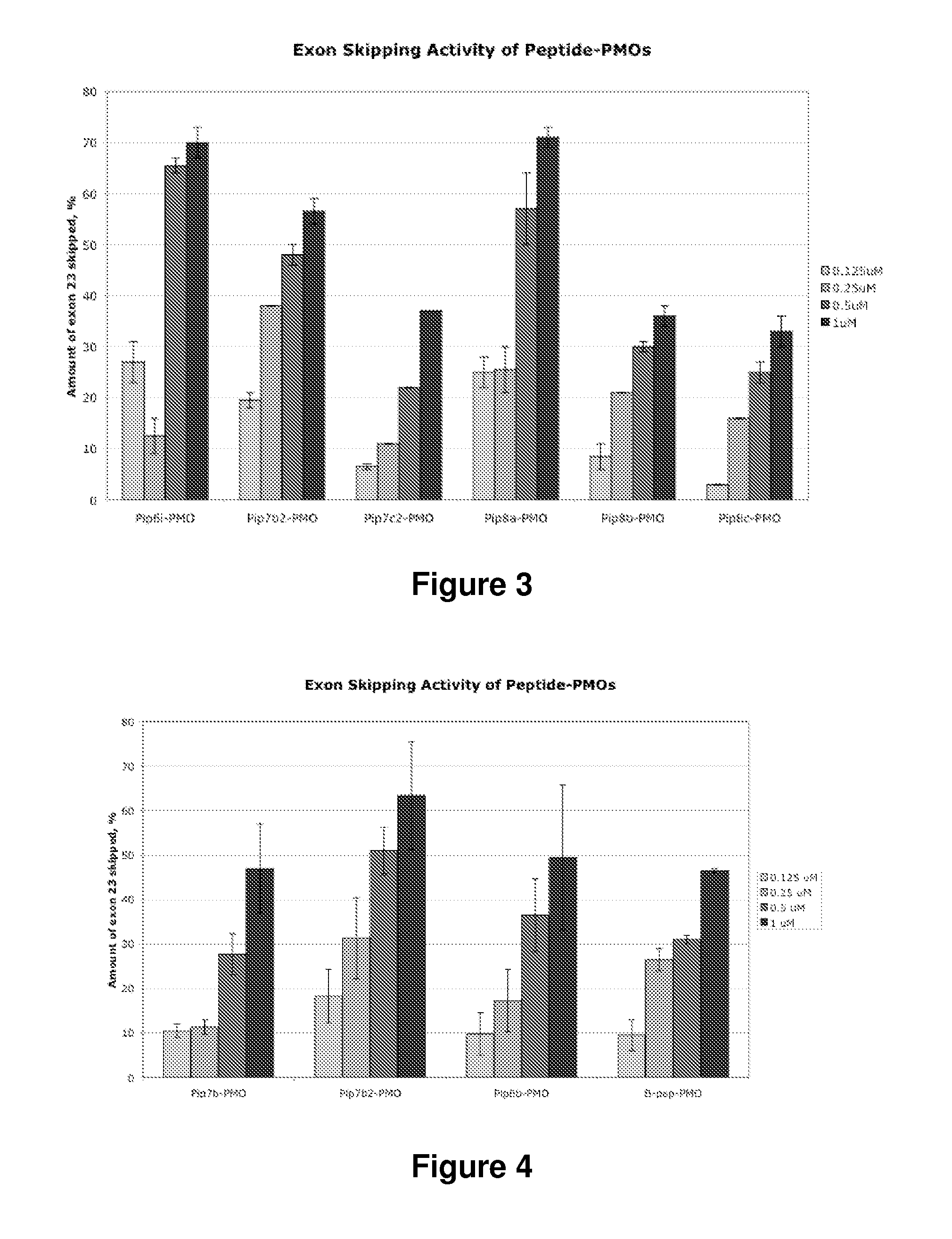
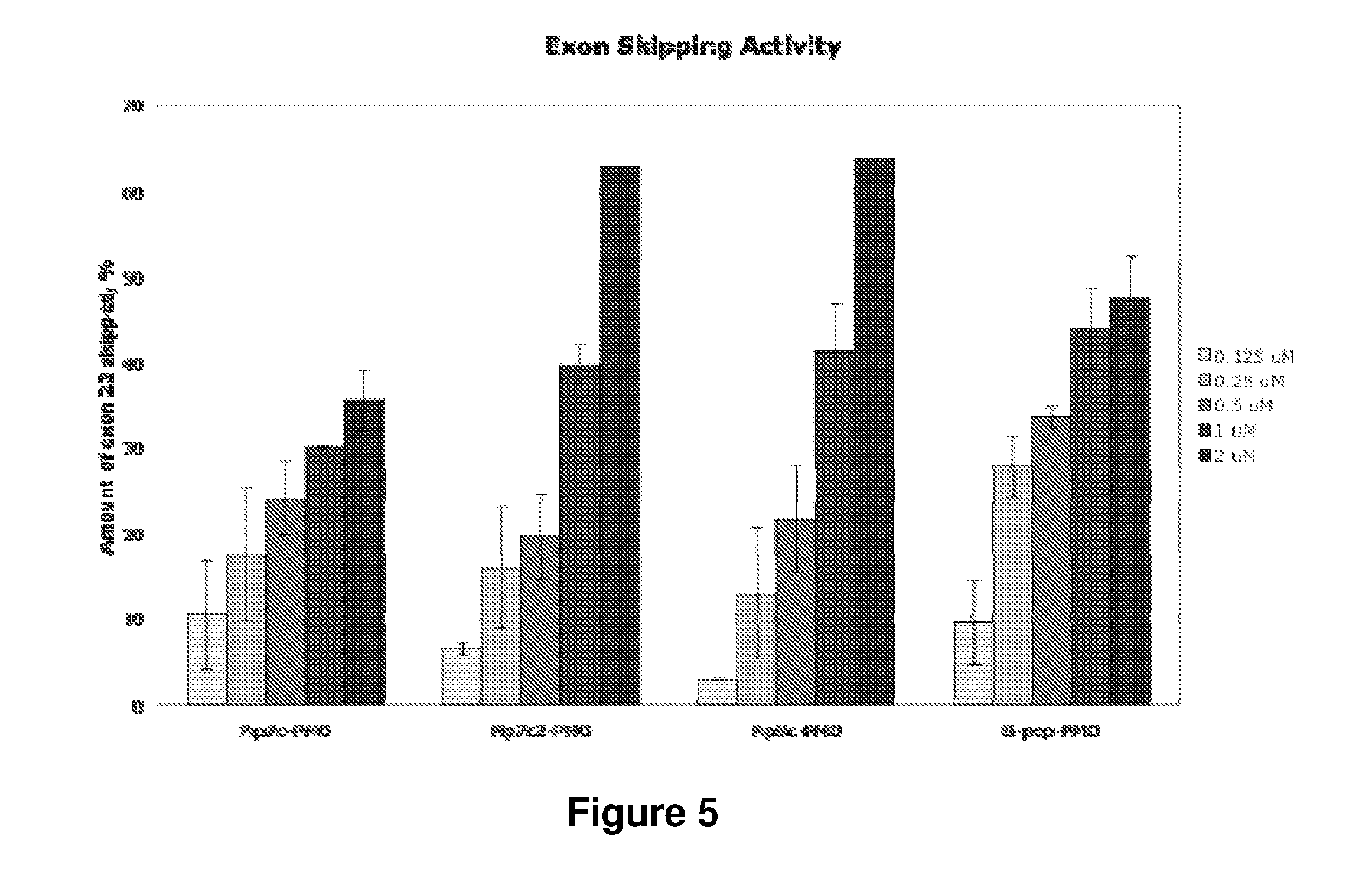
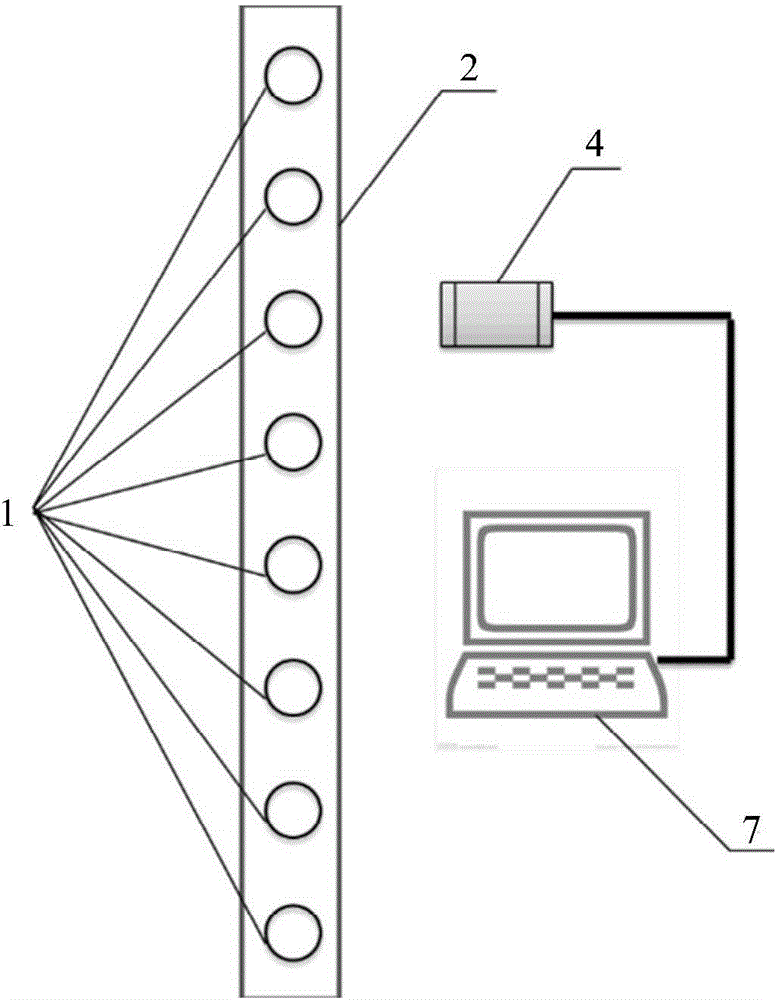
Fluorogenic dyes
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
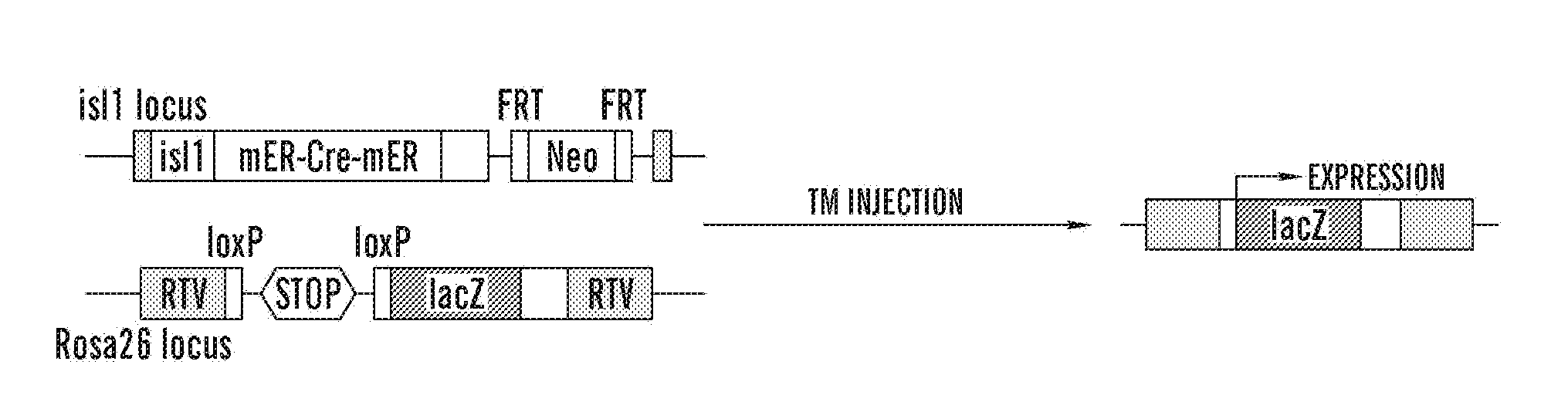
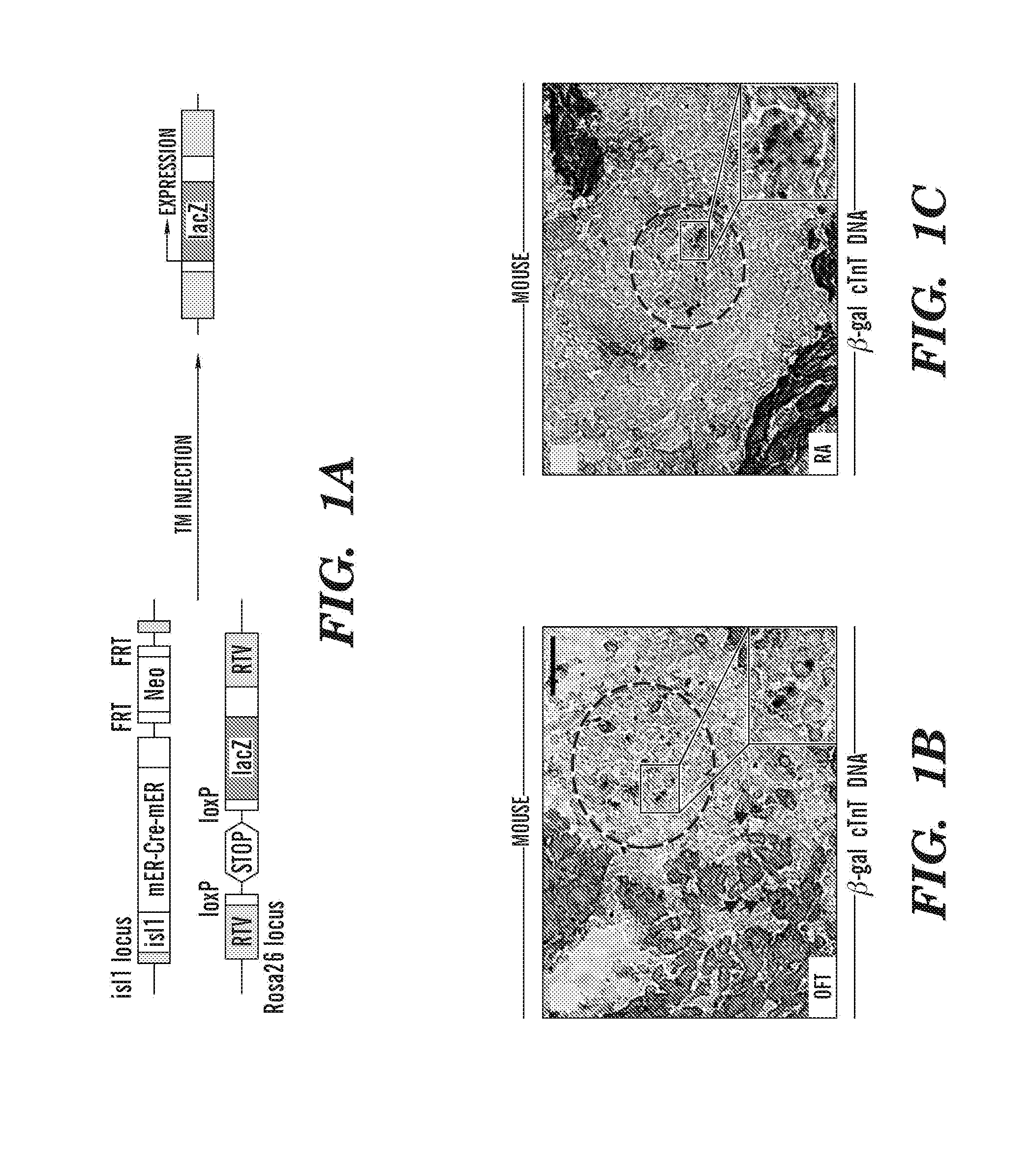
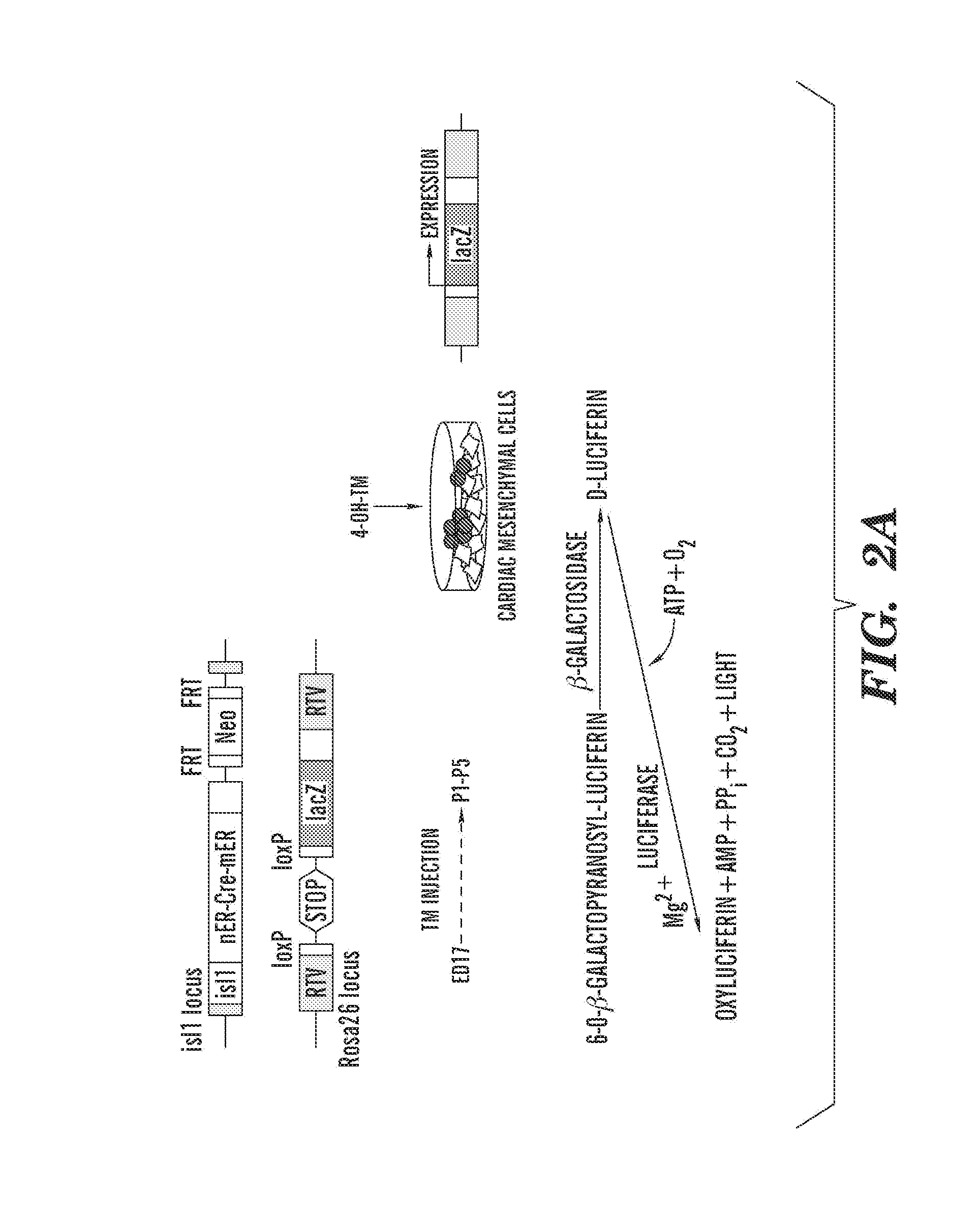
Methods for the induction of a cell to enter the islet 1+ lineage and a method for the expansion thereof
InactiveUS20110033430A1Enhancing the wnt/β-catenin pathwayIncreasing and enhancing wnt signalingBiocideGenetic material ingredientsProgenitorISL1
The present invention relates to methods for the induction and a cell to enter the Islet 1+ (Isl1+) lineage and methods for expansion of cells of islet 1+ lineage. One aspect of the present invention relates to methods to induce a cell to enter the islet 1+ lineage, and more particularly to a method to induce a cell to enter a the Isl1+ lineage to become an Isl1+ progenitor that is capable of differentiating along multiple different lineages such as a endothelial lineage, a smooth muscle lineage or a cardiac lineage. In particular, one embodiment present invention relates to methods to induce a cell to enter the Isl1+ lineage by inhibiting a wnt signalling pathway in the cell. Another aspect of the present invention relates to methods to expand a cell of the Isl1+ lineage, such as a Isl1+ progenitor by activating a wnt signalling pathway in the Isl1+ progenitor. Another aspect of the present invention relates to use of cells of the isl1+ lineage in subjects for therapeutic and preventative treatment of cardiovascular diseases.
Owner:THE GENERAL HOSPITAL CORP
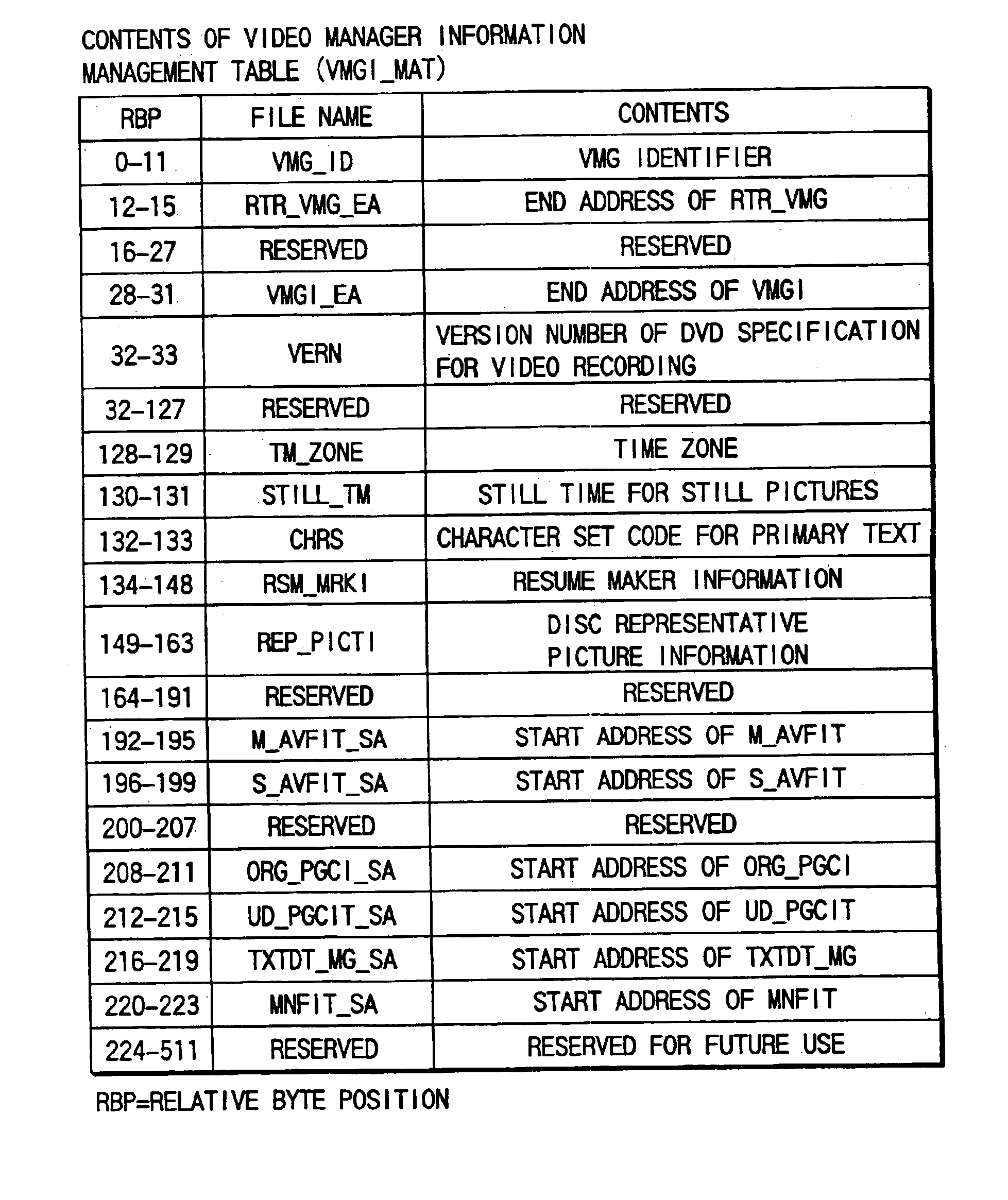
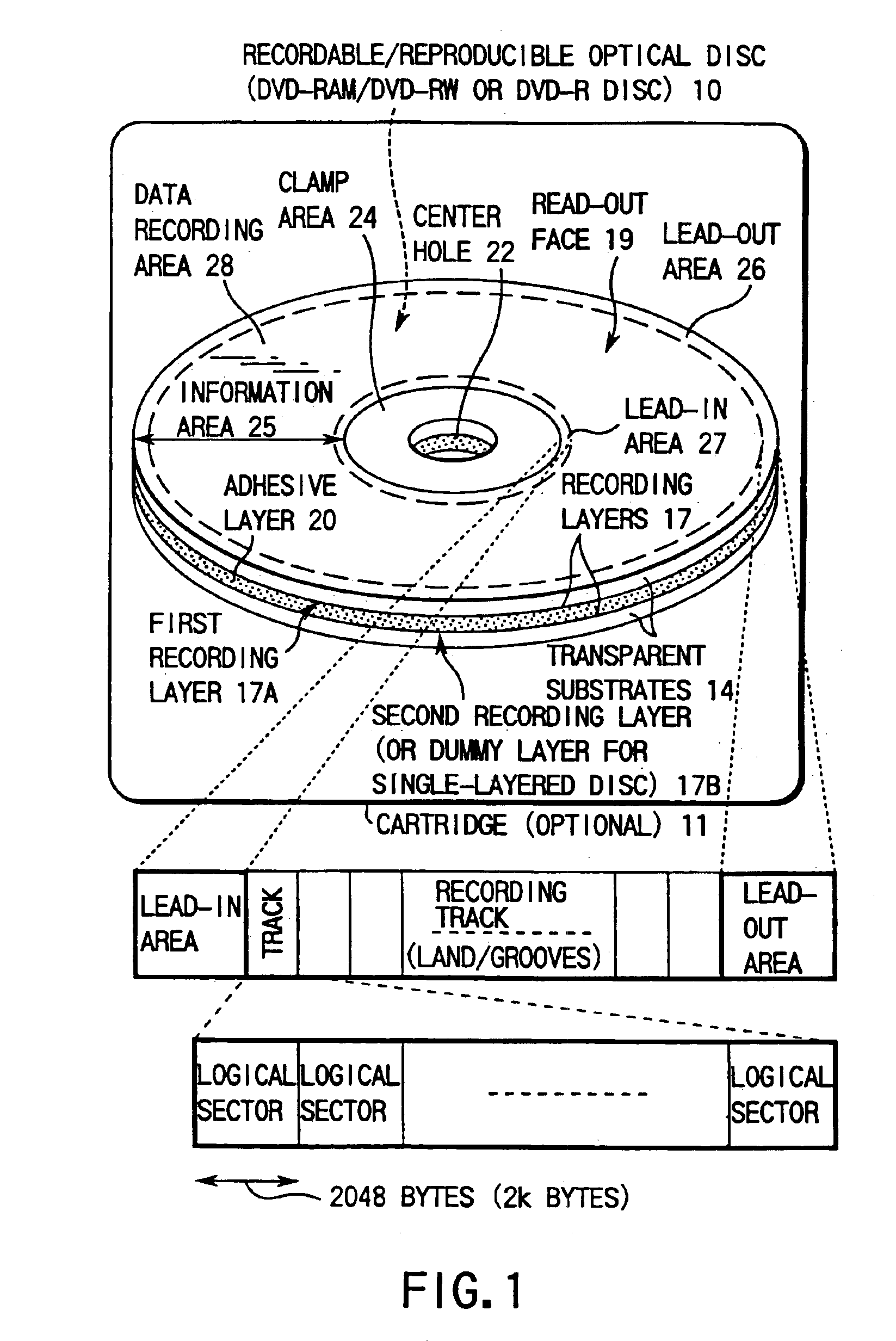
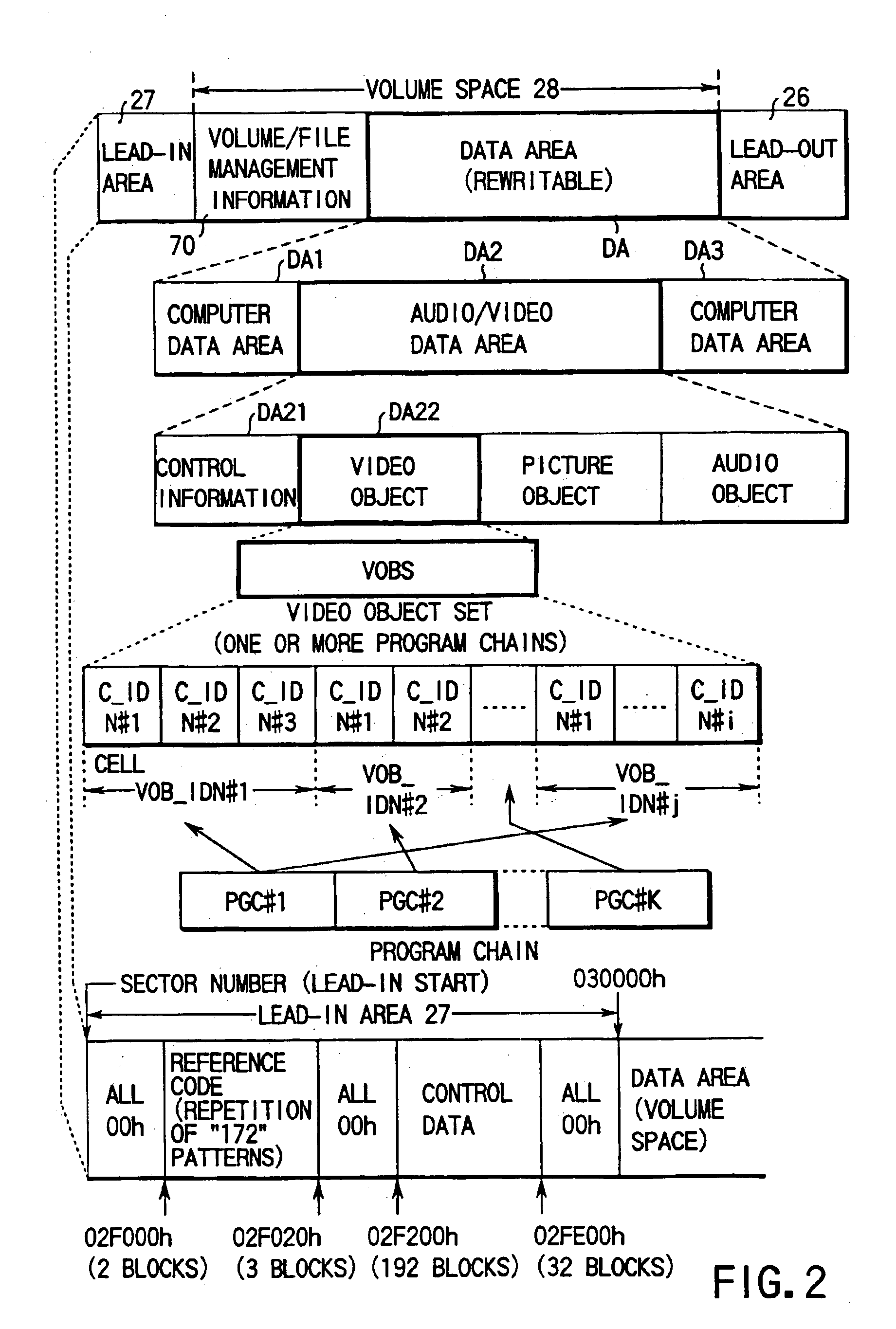
Digital video recording/playback system with entry point processing function
InactiveUS6944391B2Easy to identifyTelevision system detailsRecord information storageTime informationDigital video
This invention allows the user to insert an entry point (bookmark) at an arbitrary recording position of video data, audio data, and the like as if he or she placed a bookmark between pages of a book. Information RTR_VMG that manages recorded objects includes movie cell entry point information M_C_EPI. M_C_EPI includes entry point playback time information EP_PTM and text information PRM_TXTI that pertains to an entry point. PRM_TXTI can store text information that pertains to its contents together with type information and date information of an entry point.
Owner:NORTEL NETWORKS LTD
Membrane translocating peptide drug delivery system
InactiveUS7268214B2Facilitated DiffusionImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsLipid formationCell layer
The present invention relates to a novel membrane translocating full-length peptide sequence, fragment, motif, derivative, analog or peptidomimetic thereof (MTLPs), to nucleotide sequences coding therefor, and to compositions comprising a MTLP-active agent complex and a MTLP-active particle complex. The MTLP or the nucleotide sequence coding therefor enhance movement of the active agent or of the active particle across a lipid membrane. More particularly, the present invention relates to a MTLP-active agent complex and a MTLP-active particle complex, wherein the MTLP enhances uptake of the active agent into a cell, into or out of an intracellular compartment and across a cell layer. Methods of making and methods of using MTLPs also are included.
Owner:MERRION RES I
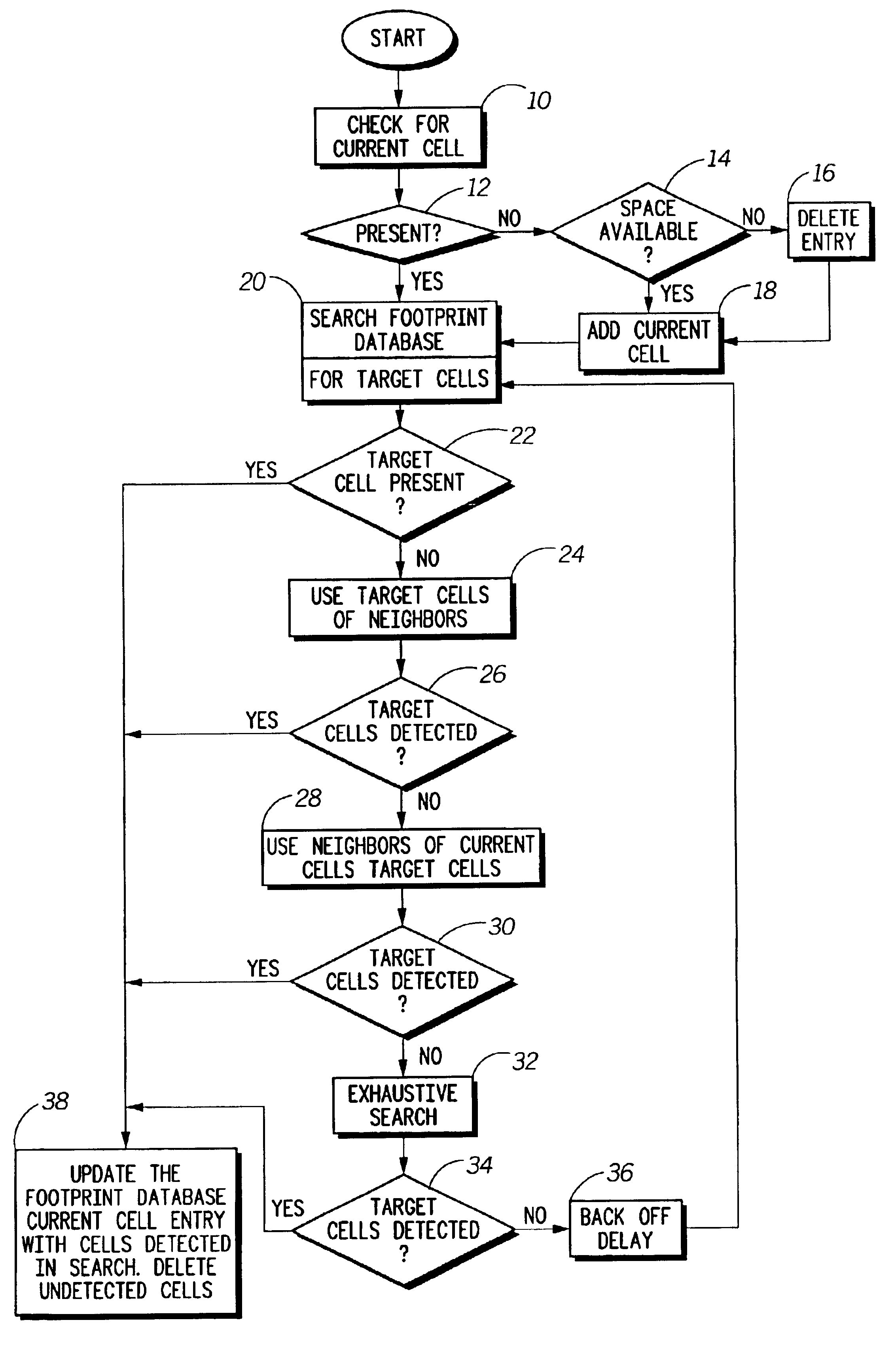
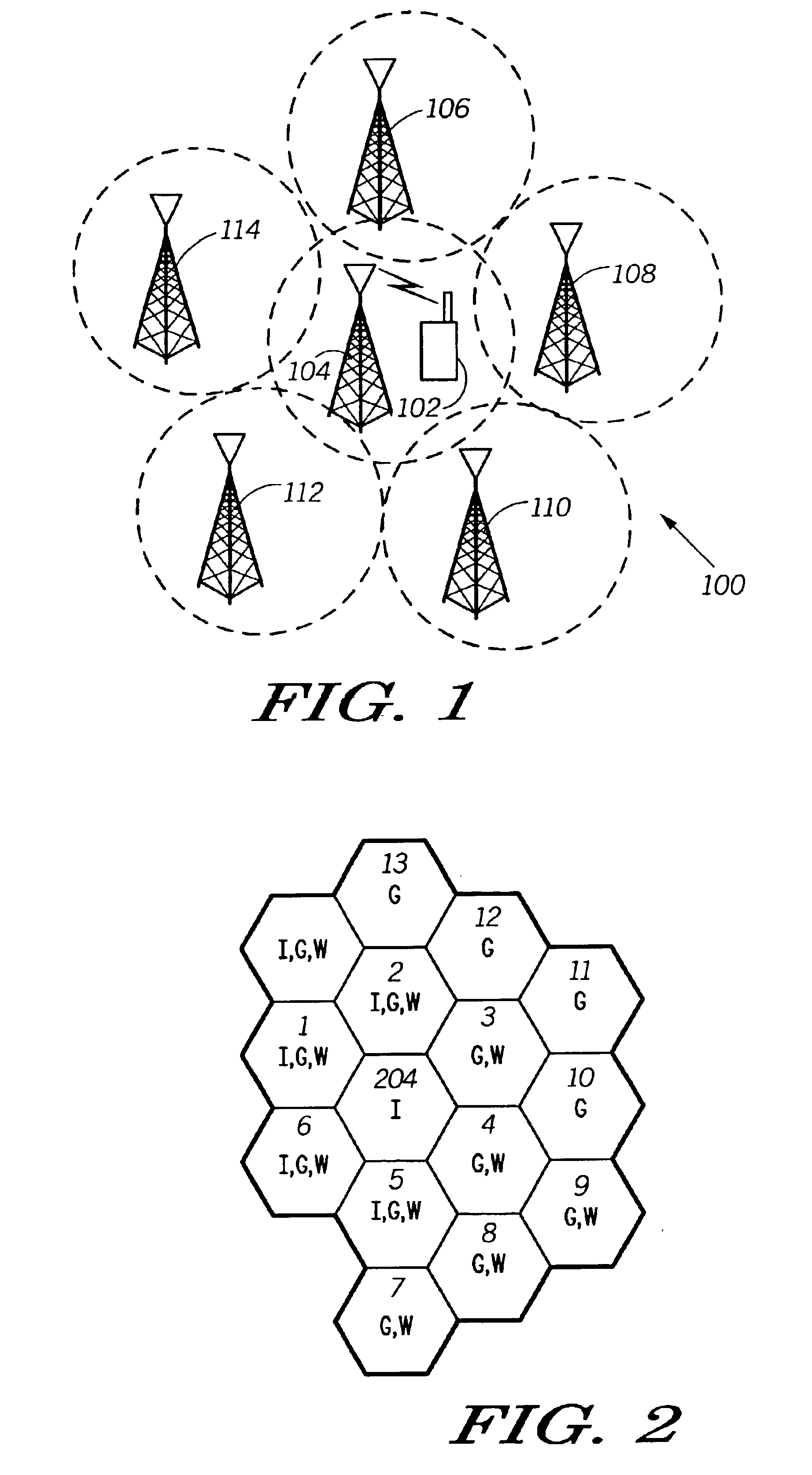
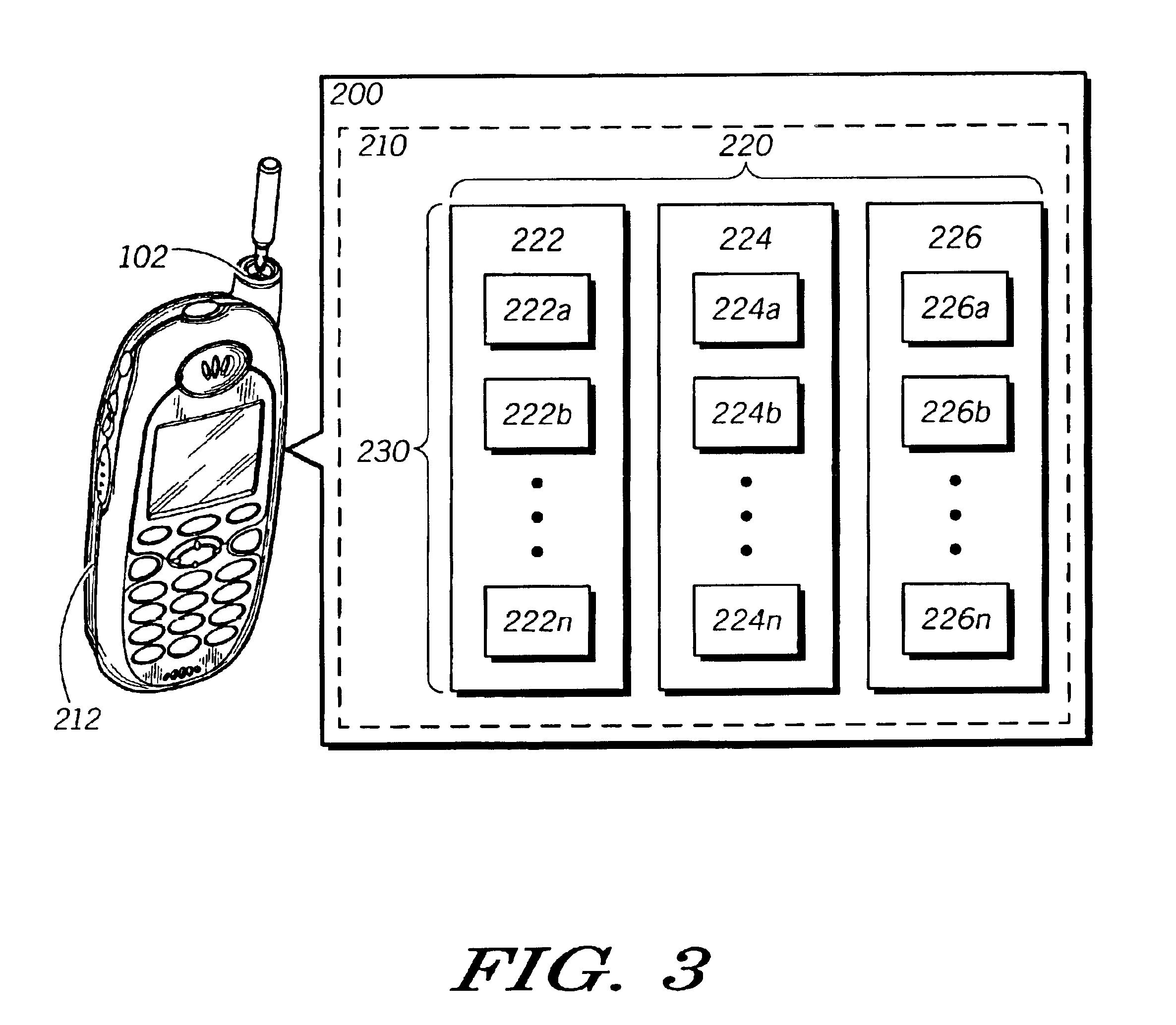
Method and system for efficiently using a mobile unit for switching between communications systems
InactiveUS6889049B2Efficient switchingAssess restrictionRadio/inductive link selection arrangementsCommunication unitCommunications system
The invention provides a method and apparatus for allowing a multi-mode mobile communication unit (102) to switch efficiently between communication systems. The method includes creating and adaptively updating a footprint database (210) relating the coverage of the multiple communication systems. The footprint database (210) comprises a plurality of system lists (220). The system lists (220) each include a plurality of cell entries (222) linking cells of a particular system to cells in other systems. Each of the plurality of cell entries is identified by a cell frequency (342) and a color code (344). The method further comprises the step of installing and dynamically updating the footprint database (210) on the mobile unit (102). Accordingly, an apparatus of the invention includes a mobile unit (102) having the aforementioned database installed thereon. The mobile unit (102) additionally includes processing tools (212) for accessing a user selected system list upon receiving a user request and for locating the selected system by cycling through cell entries in the system lists.
Owner:GOOGLE TECH HLDG LLC
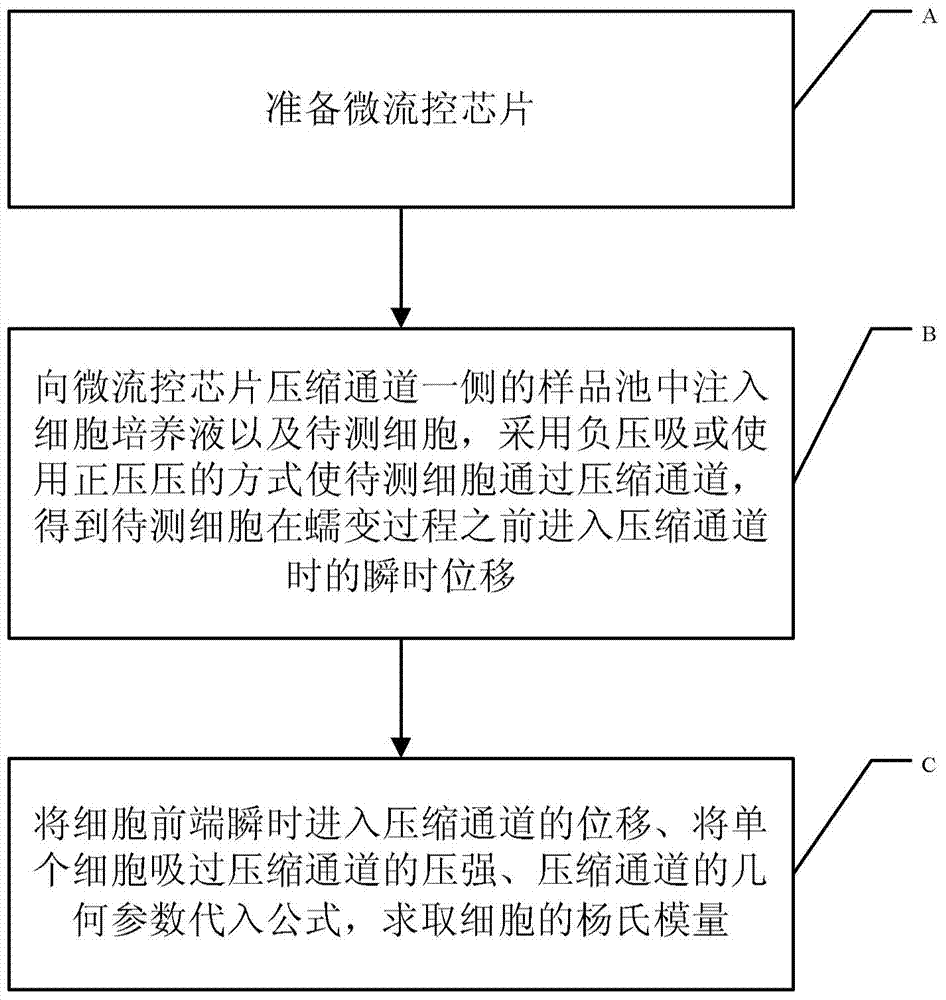
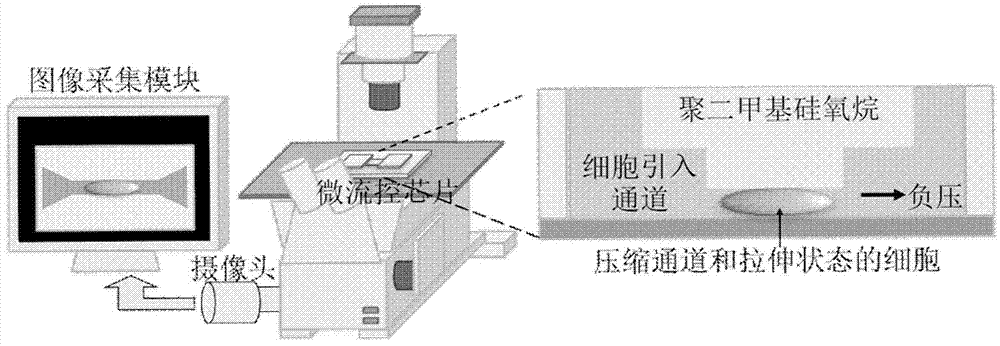
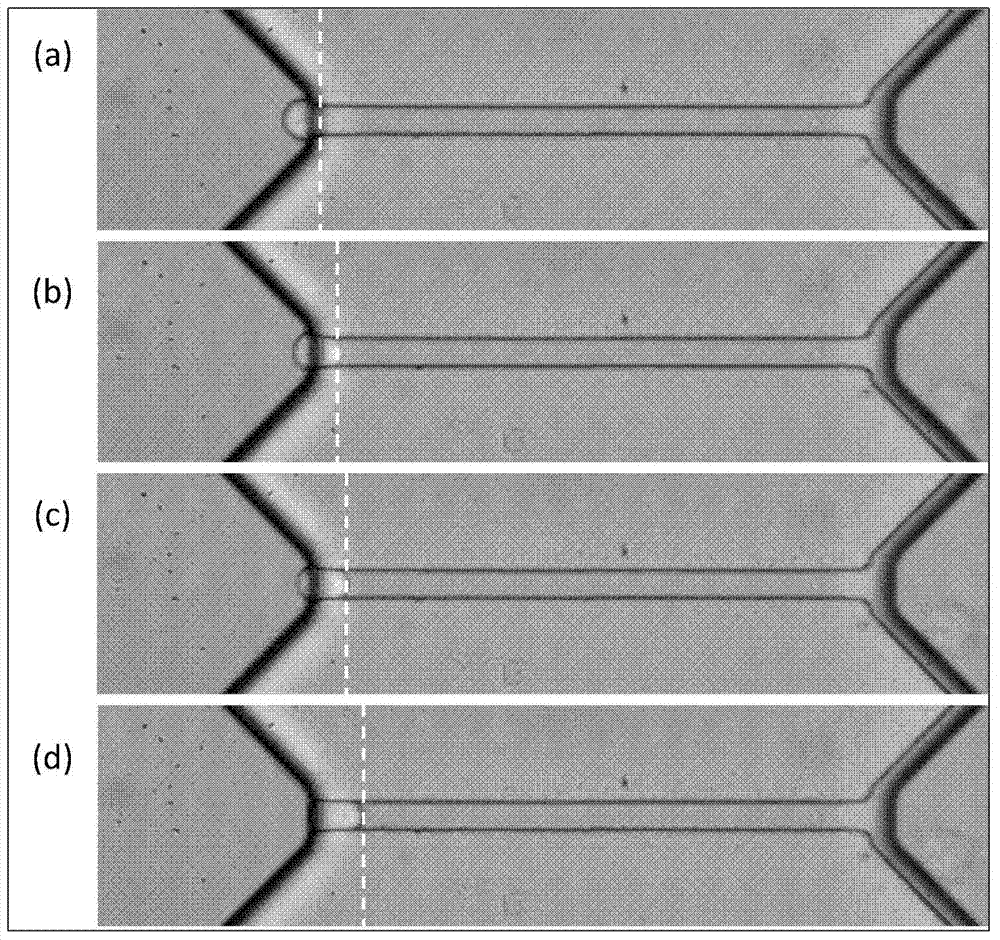
Method for measuring Young modulus of single cell based on micro-fluidic technology
ActiveCN103674813AImprove portabilityLow costIndividual particle analysisMechanical modelsYoung's modulus
The invention provides a method for measuring Young modulus of single cell based on micro-fluidic technology. According to the method, a to-be-measured cell is equivalent to an isotropous viscoelastic body, with an equivalent mechanical model of the to-be-measured cell of entering and running through a compression channel, based on a relation of a displacement of the front end of the to-be-measured of instantly entering the compression channel, the size of the cell, the Yong modules, the intensity of pressure, and the geometric parameters of the compression channel, the measurement for the Young modulus of the to-be-measured cell is realized.
Owner:INST OF ELECTRONICS CHINESE ACAD OF SCI
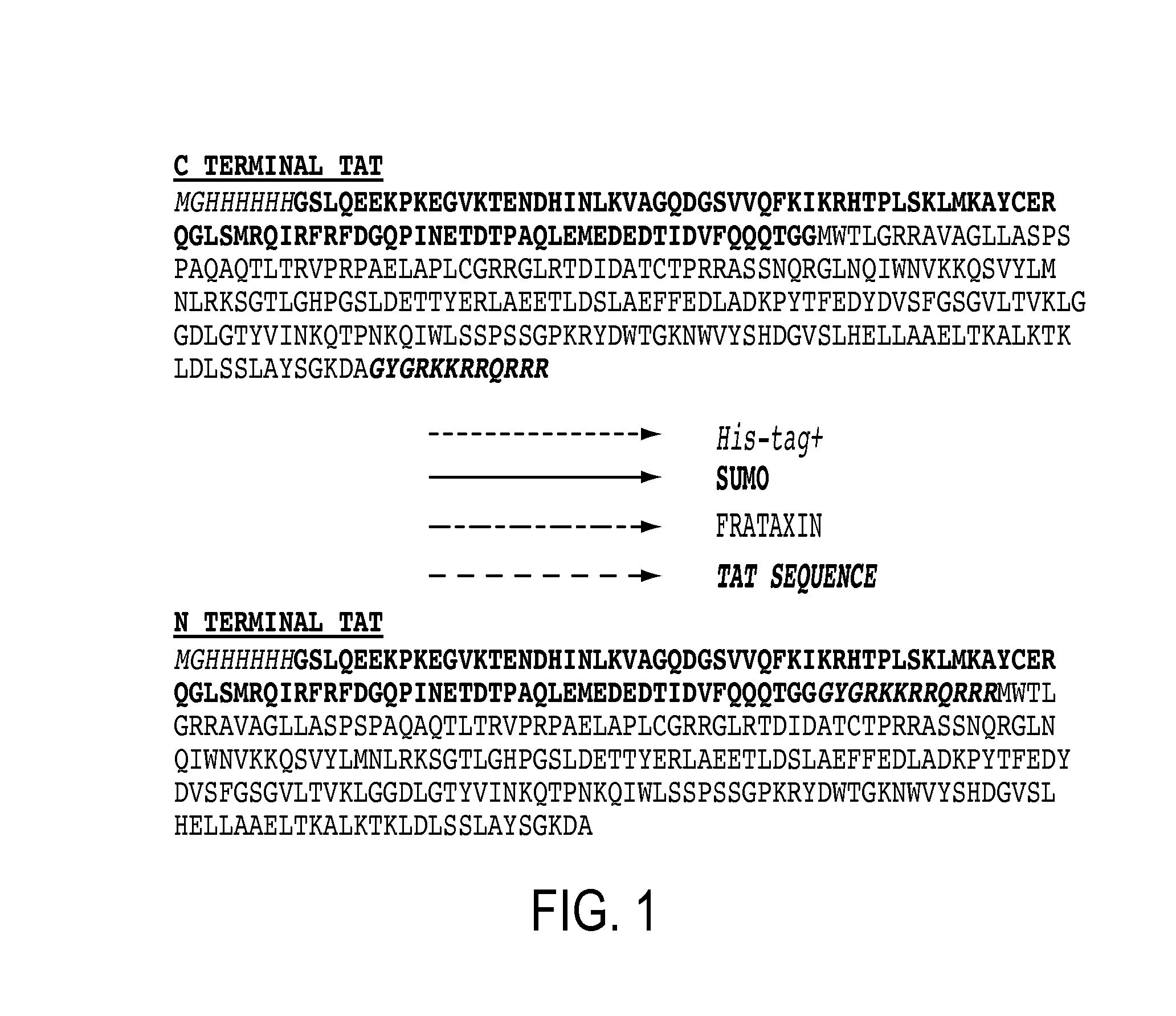
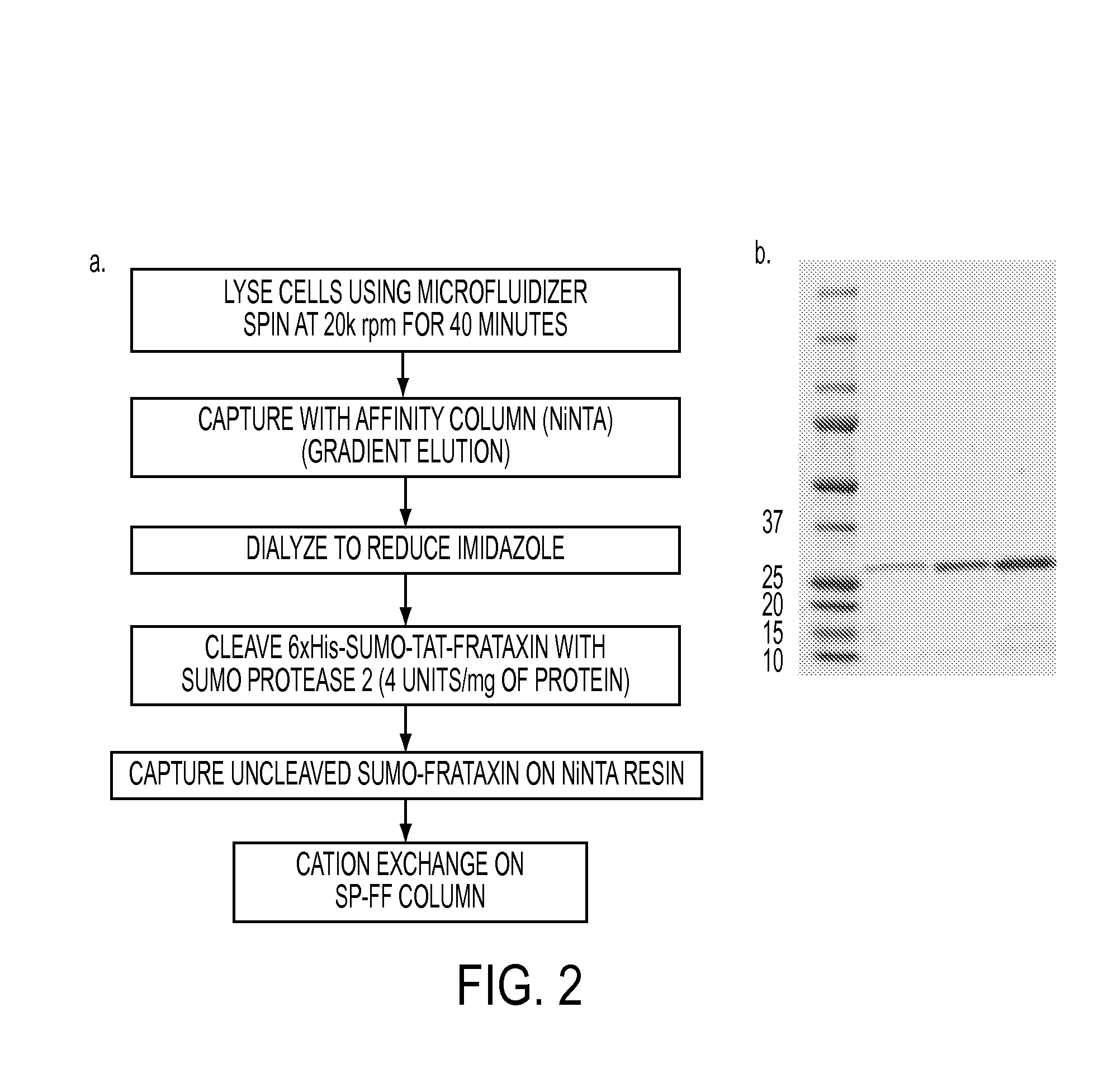
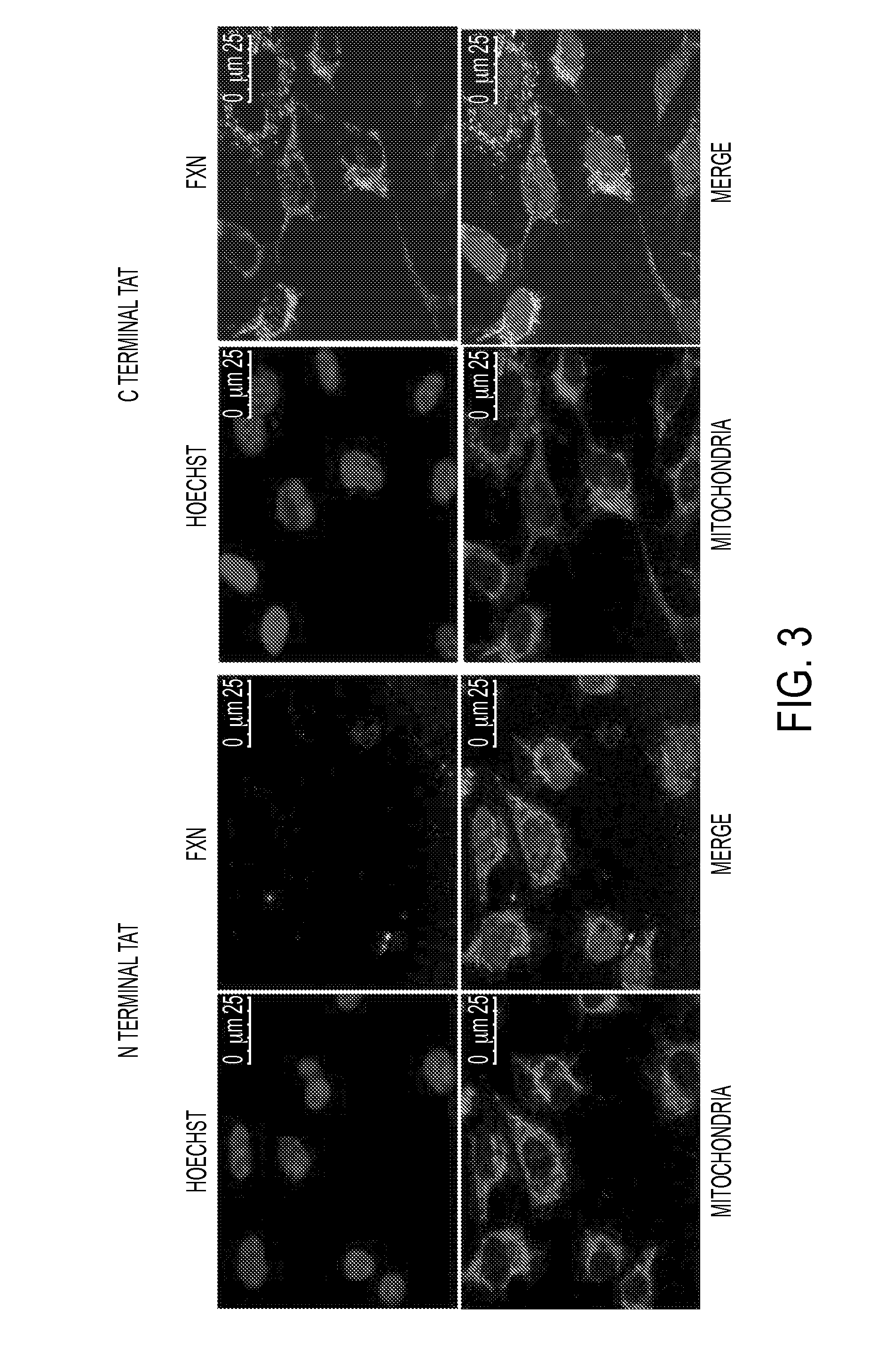
Mitochondrial targeting and therapeutic use thereof
ActiveUS20140135275A1Enhance cell viabilityCompounds screening/testingBacteriaFrataxinMitochondrion membrane
The present invention provides, among other things, compositions and methods for treatment of Friedrich's Ataxia based on effective targeting of a therapeutic moiety to mitochondria that can substitute for natural FXN protein activity or rescue one or more phenotypes or symptoms associated with frataxin-deficiency. In some embodiments, the present invention provides a targeted therapeutic comprising a therapeutic moiety, which is a polypeptide having an N-terminus and a C-terminus, a mitochondrial targeting sequence associated with the therapeutic moiety at the N-terminus, and a mitochondrial membrane-penetrating peptide associated with the therapeutic moiety at the C-terminus, wherein the therapeutic moiety is targeted to mitochondria upon cellular entry.
Owner:TAKEDA PHARMA CO LTD
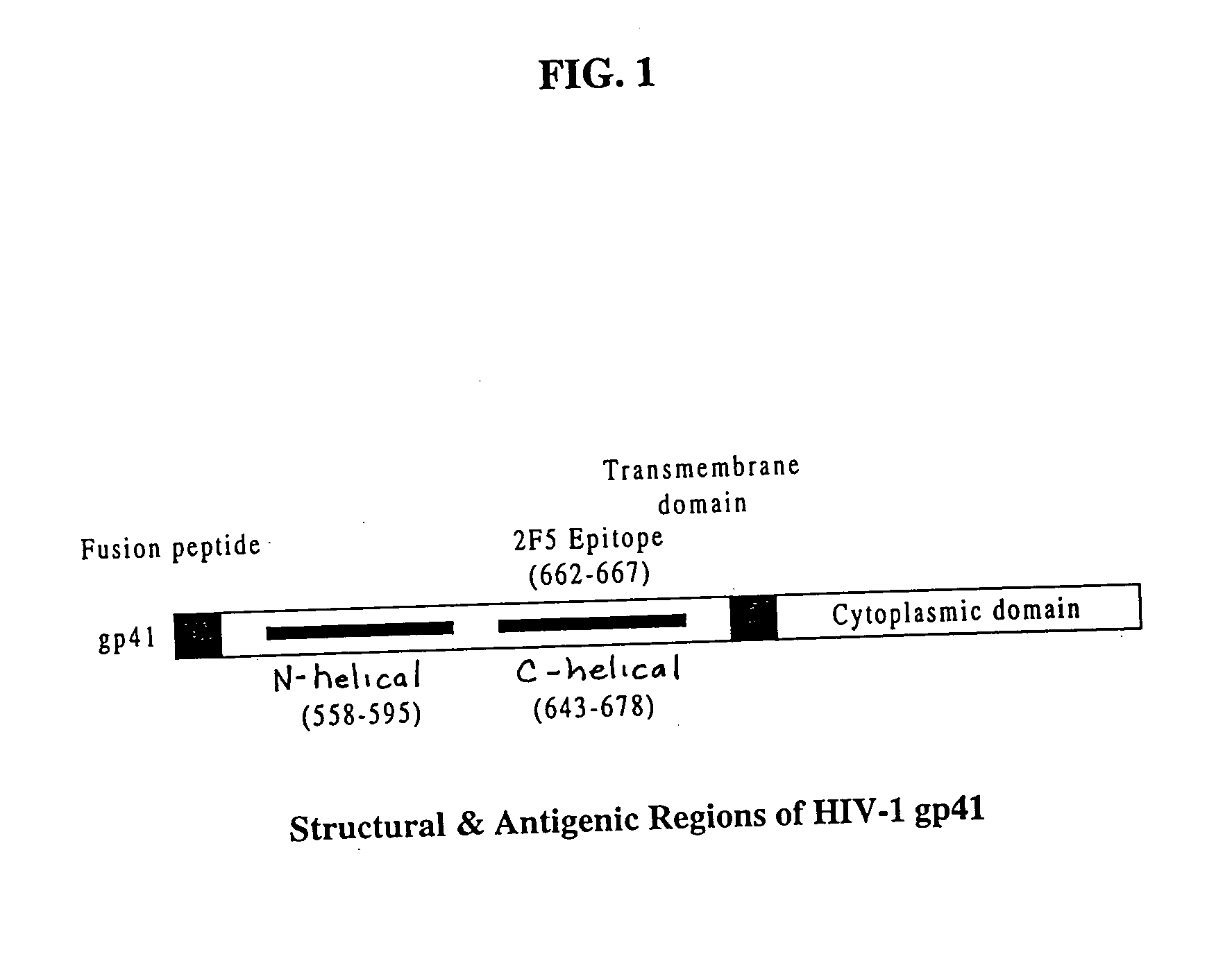
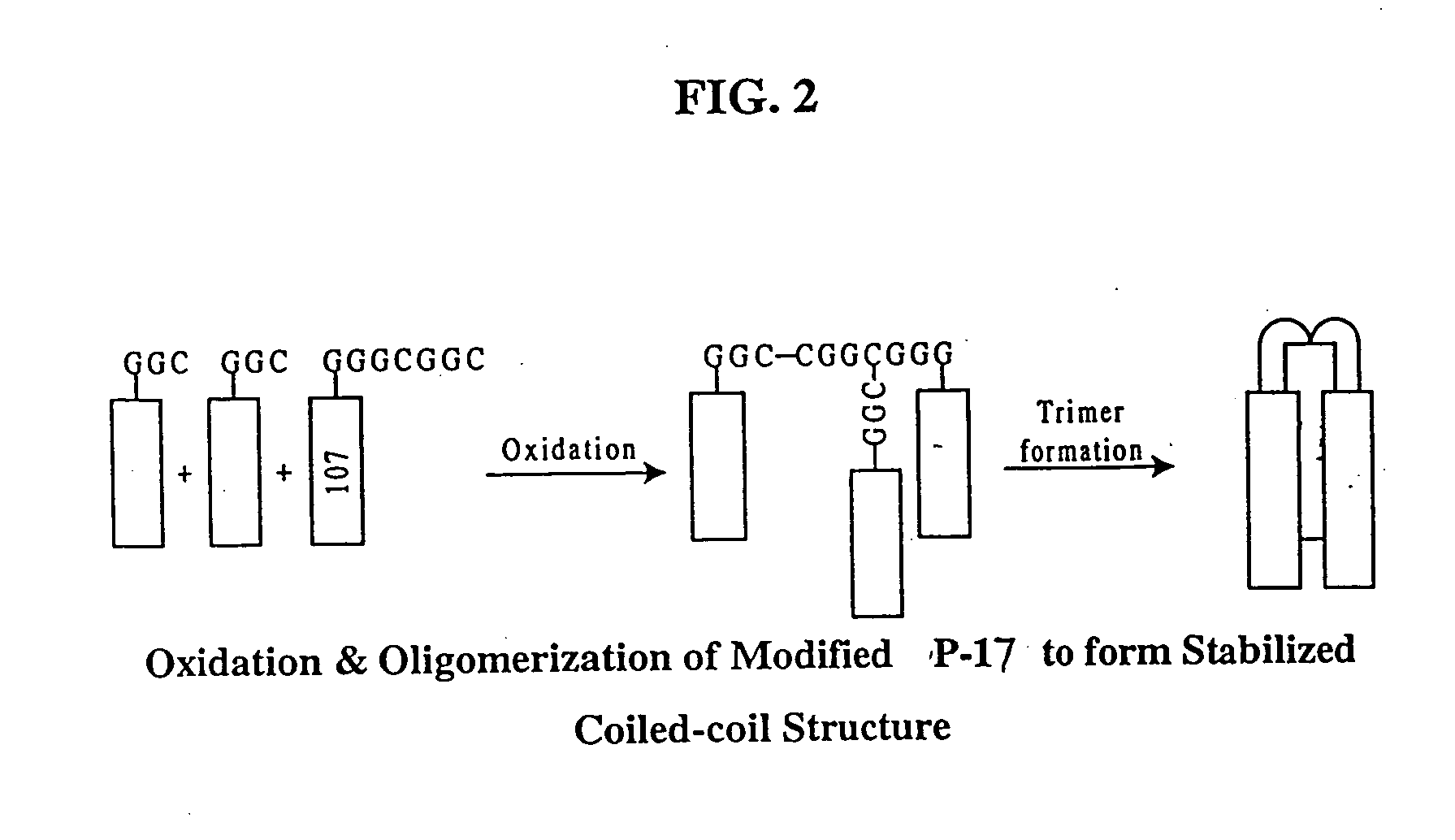
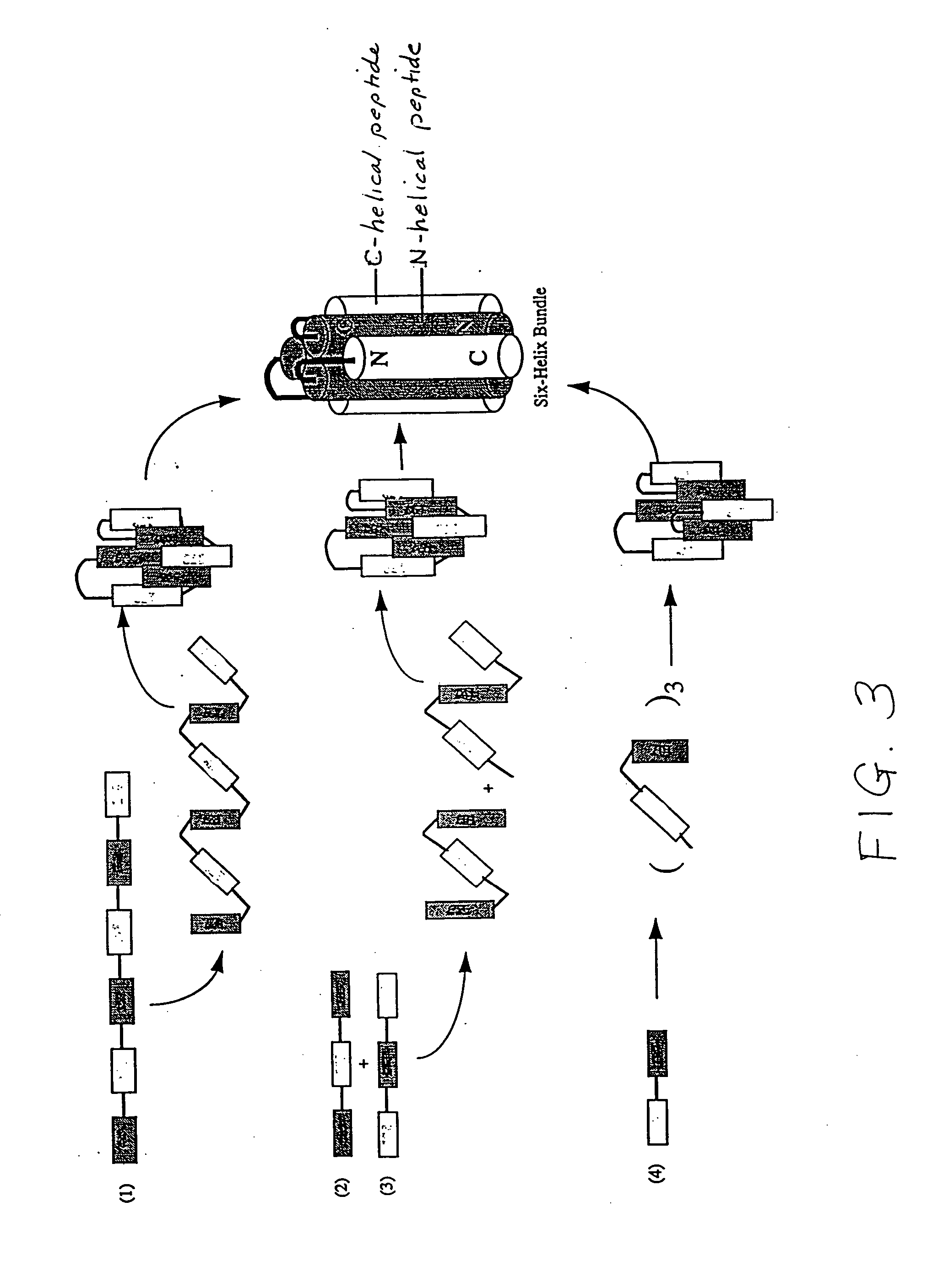
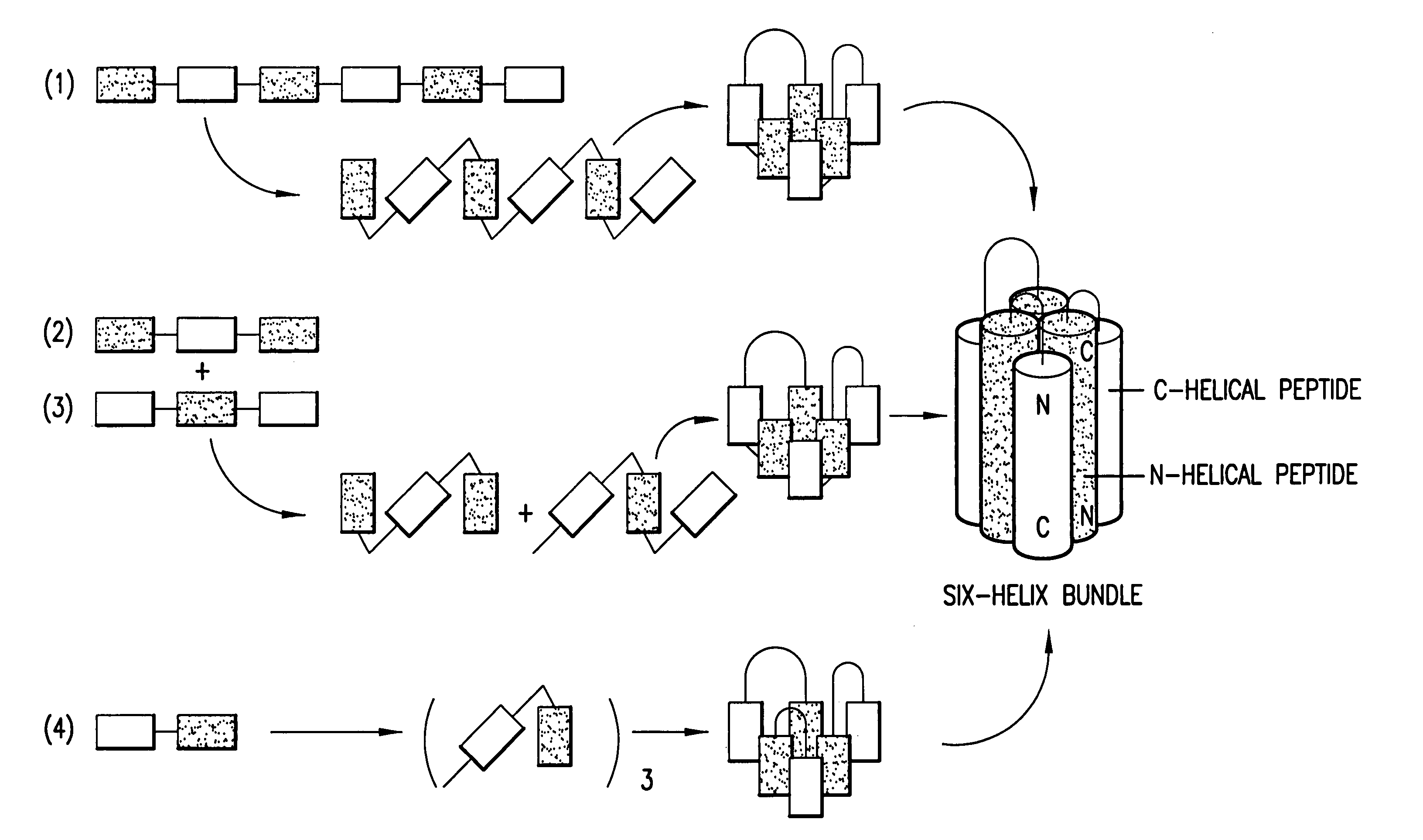
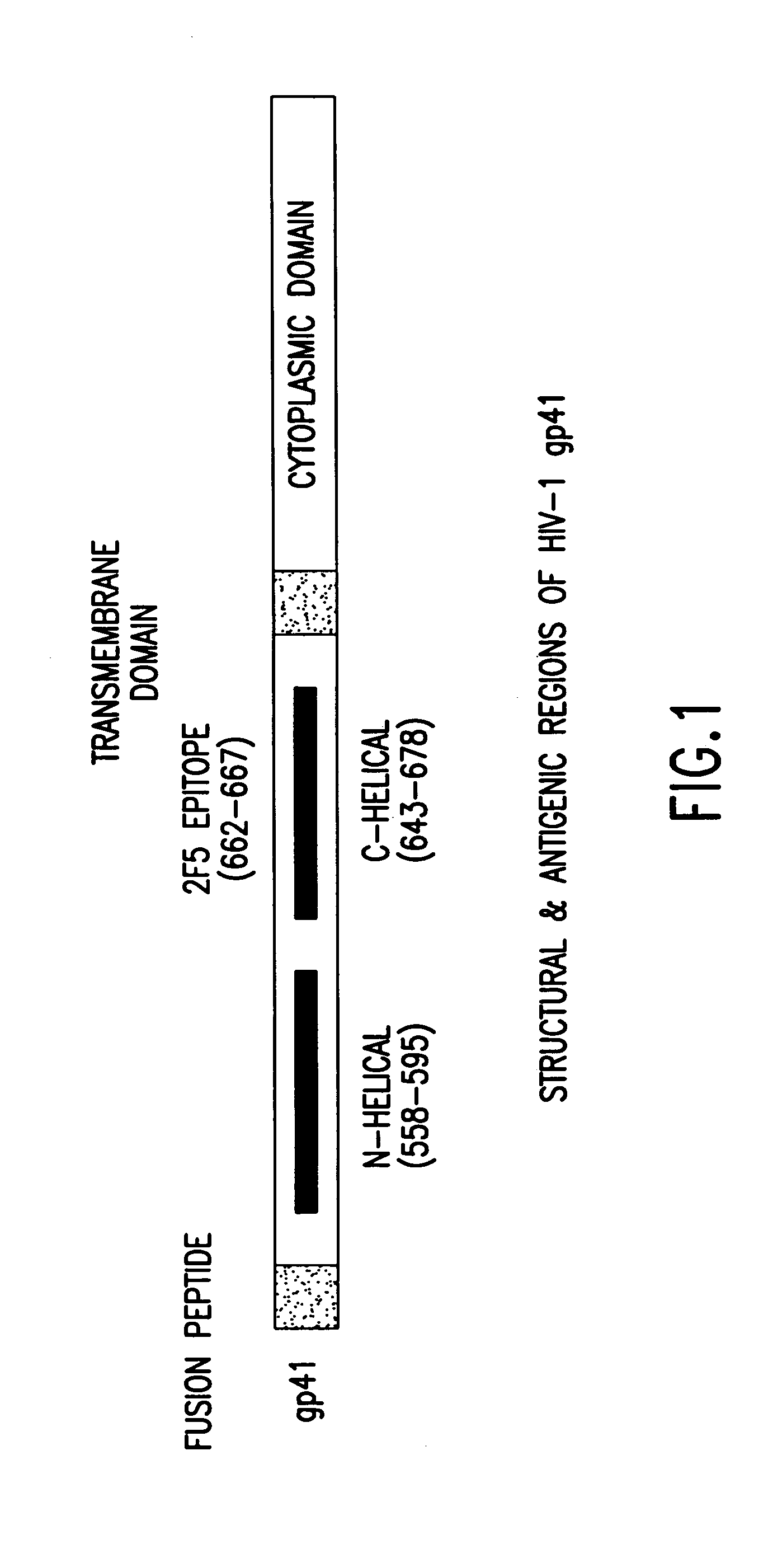
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
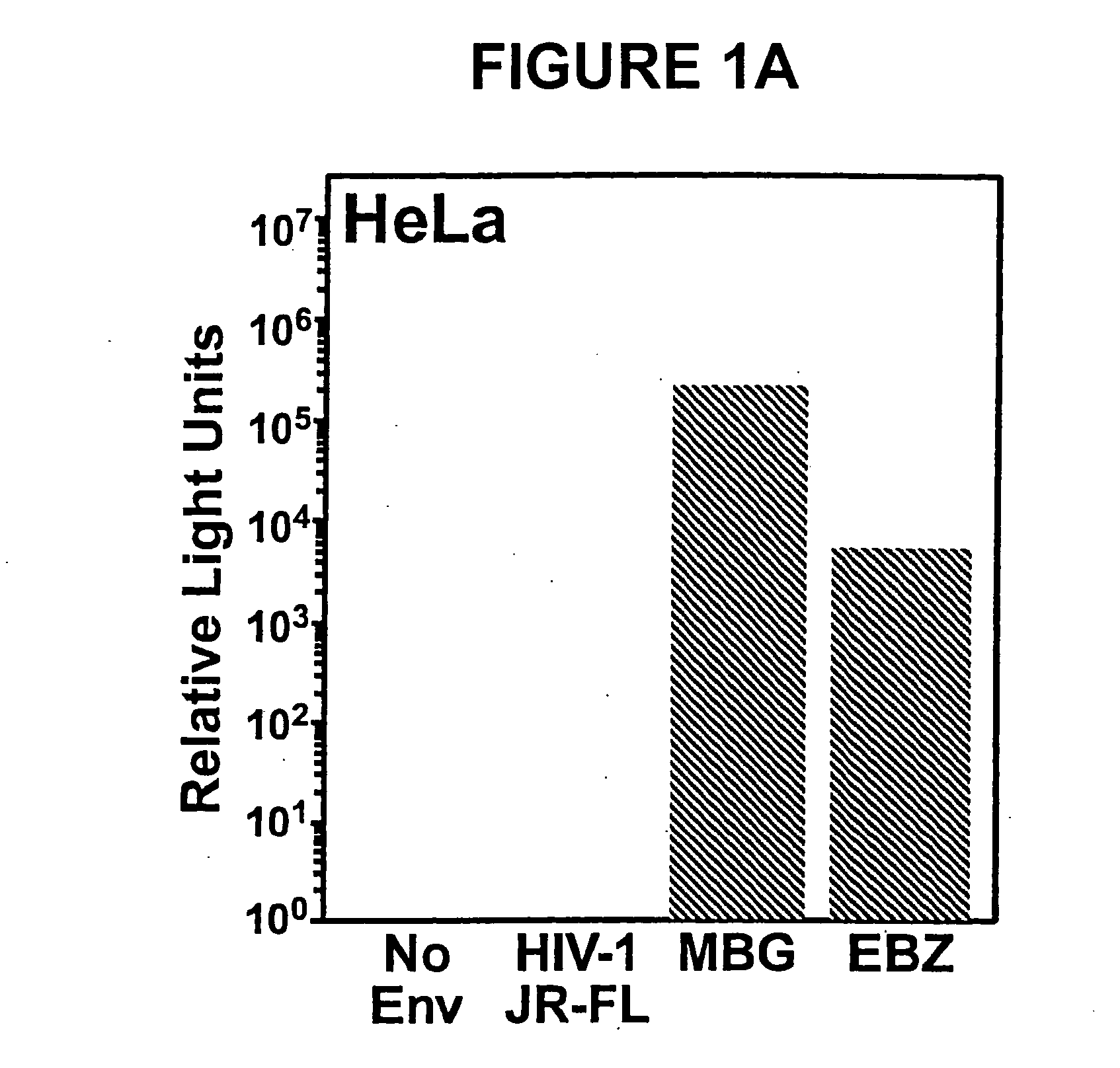
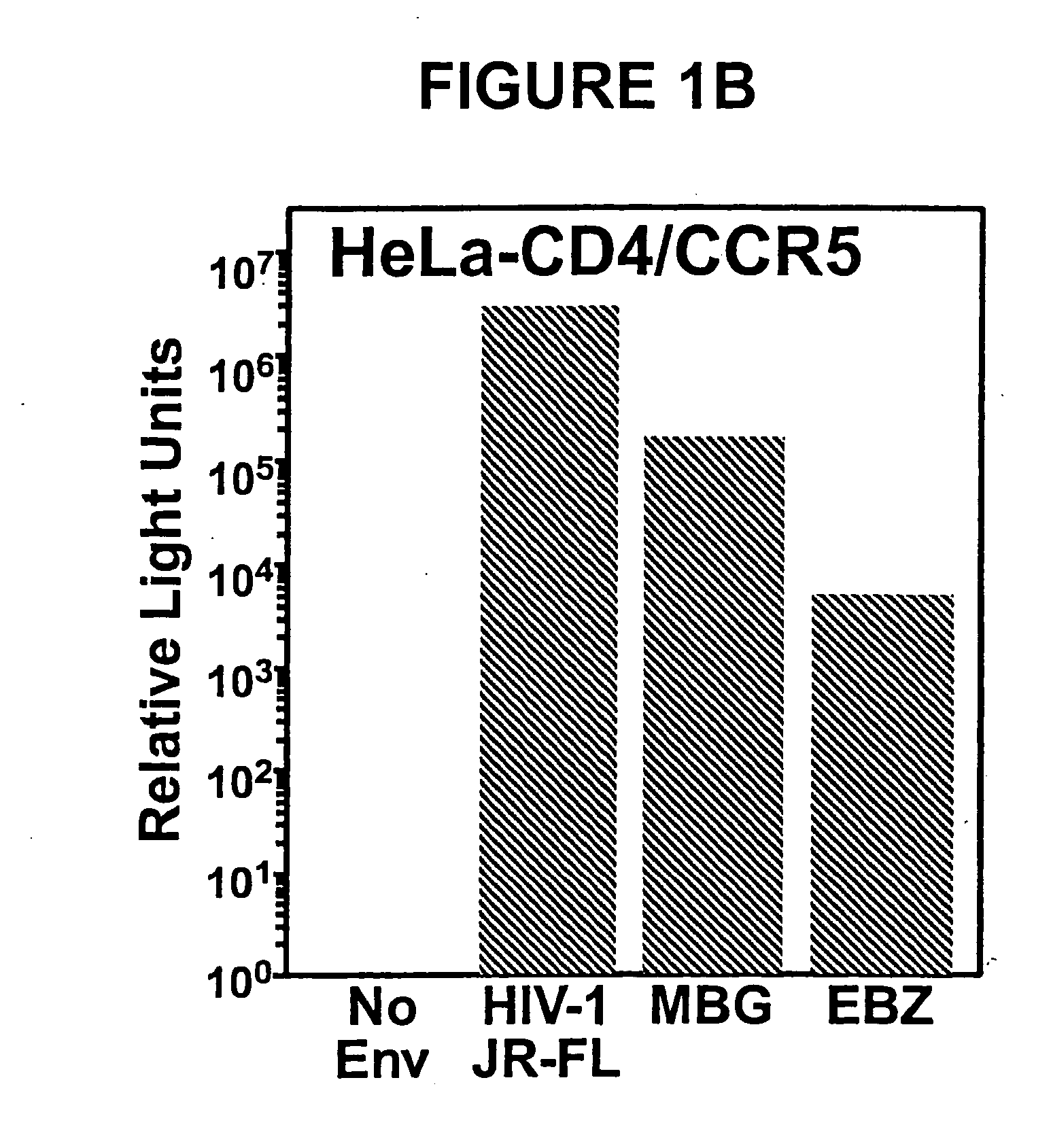
The present invention is directed to the induction and characterization of a humoral immune response targeting "entry-relevant" gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against "entry relevant" gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
The present invention is directed to the induction and characterization of a humoral immune response targeting “entry-relevant” gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against “entry relevant” gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
Immunogenic peptides of influenza virus
Peptides and polypeptides that elicit immunogenic responses in a mammal; especially neutralizing antibodies, against human and avian influenza strains H1N1, H3N2, H5N1 and H7N7 are disclosed Immunogenic compositions including these peptides, and polypeptides are also provided. Compositions including these peptides and polypeptides with or without adjuvants are disclosed. Nucleic acids and expression cassettes encoding these peptides and polypeptides are also disclosed. Methods of inhibiting infection by influenza, with or without cell entry, are also disclosed using these peptides and polypeptides.
Owner:UNITED STATES OF AMERICA
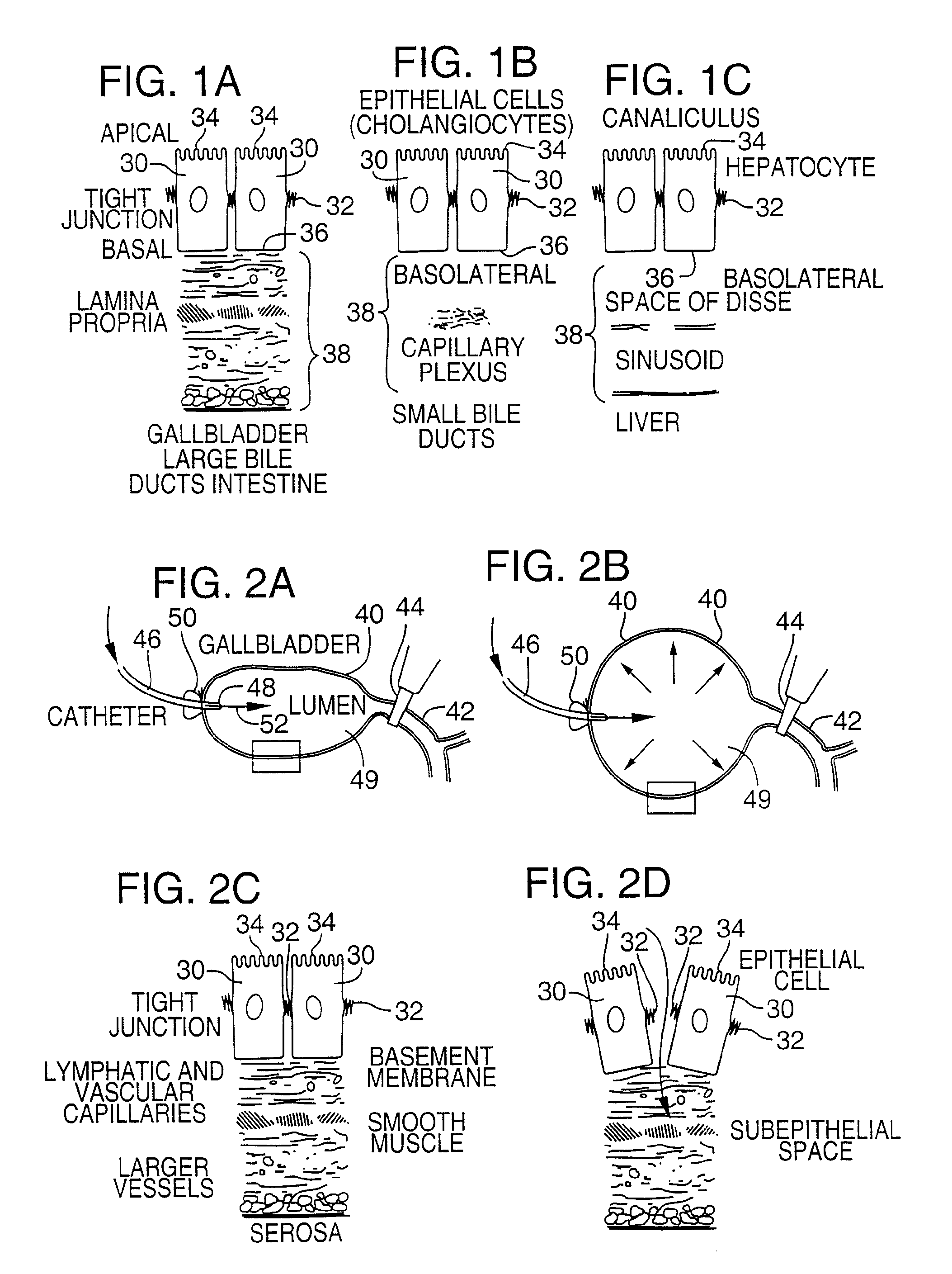
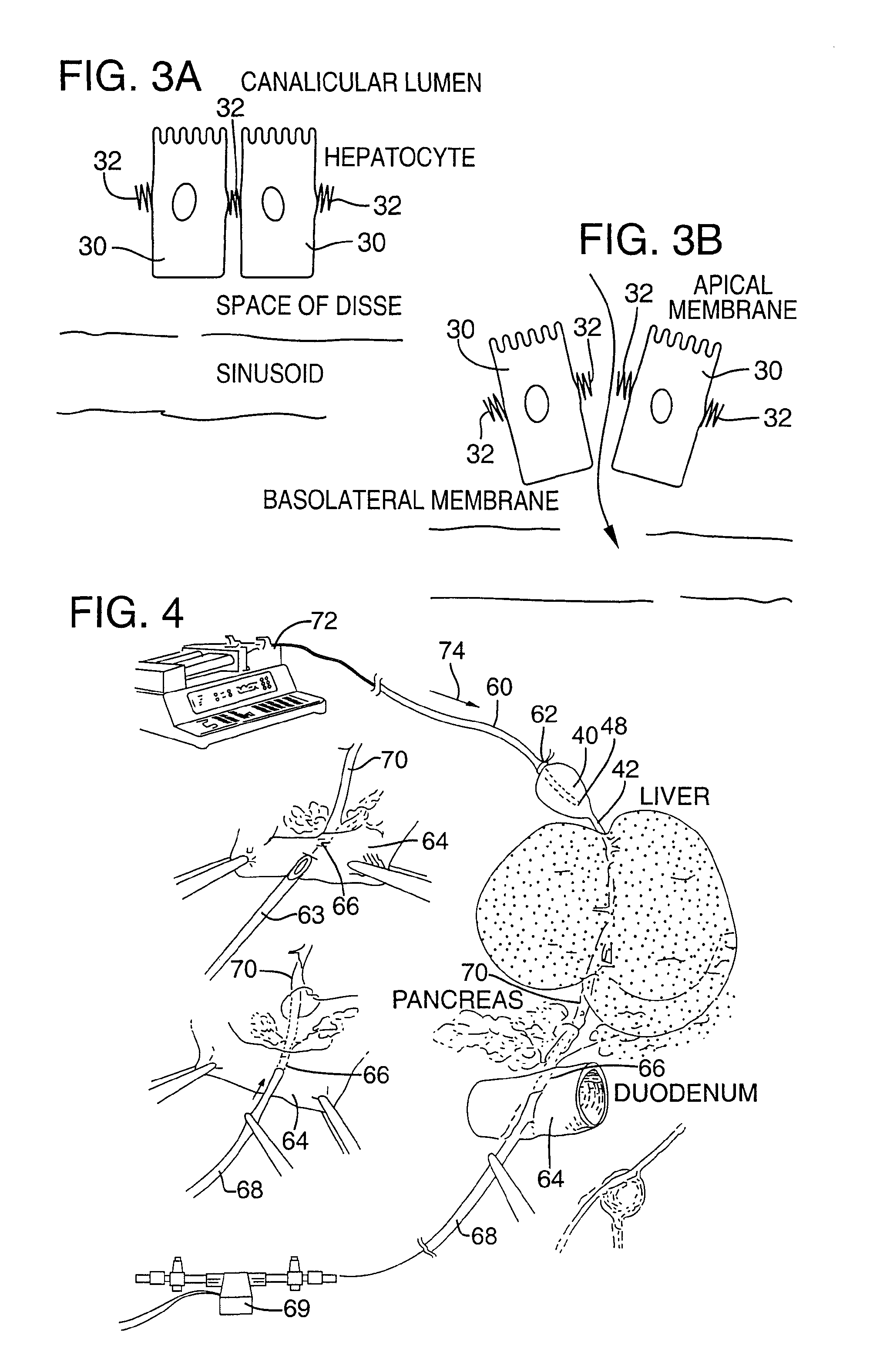
Method for pressure mediated selective delivery of therapeutic substances and cannula
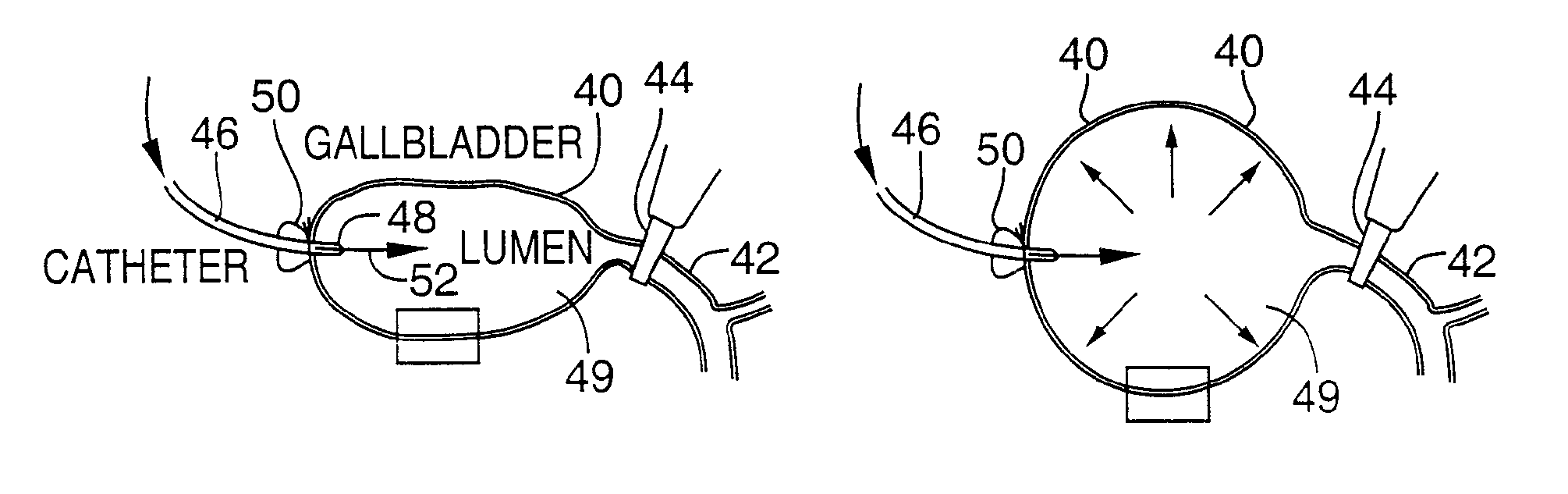
Methods and devices are disclosed for selective delivery of therapeutic substances to specific histologic or microanatomic areas of organs. Introduction of the therapeutic substance into a hollow organ space (such as an hepatobiliary duct or the gallbladder lumen) at a controlled pressure, volume or rate allows the substance to reach a predetermined cellular layer (such as the ephithelium or sub-epithelial space). The volume or flow rate of the substance can be controlled so that the intralumenal pressure reaches a predetermined threshold level beyond which subsequent subepithelial delivery of the substance occurs. Alternatively, a lower pressure is selected that does not exceed the threshold level, so that delivery occurs substantially only to the epithelial layer. Such site specific delivery of therapeutic agents permits localized delivery of substances (for example to the interstitial tissue of an organ) in concentrations that may otherwise produce systemic toxicity. Occlusion of venous or lymphatic drainage from the organ can also help prevent systemic administration of therapeutic substances, and increase selective delivery to superficial epithelial cellular layers. Delivery of genetic vectors can also be better targeted to cells where gene expression is desired. The access device comprises a cannula with a wall piercing tracar within the lumen. Two axially spaced inflatable balloons engage the wall securing the cannula and sealing the puncture site. A catheter equipped with an occlusion balloon is guided through the cannula to the location where the therapeutic substance is to be delivered.
Owner:UNITED STATES OF AMERICA
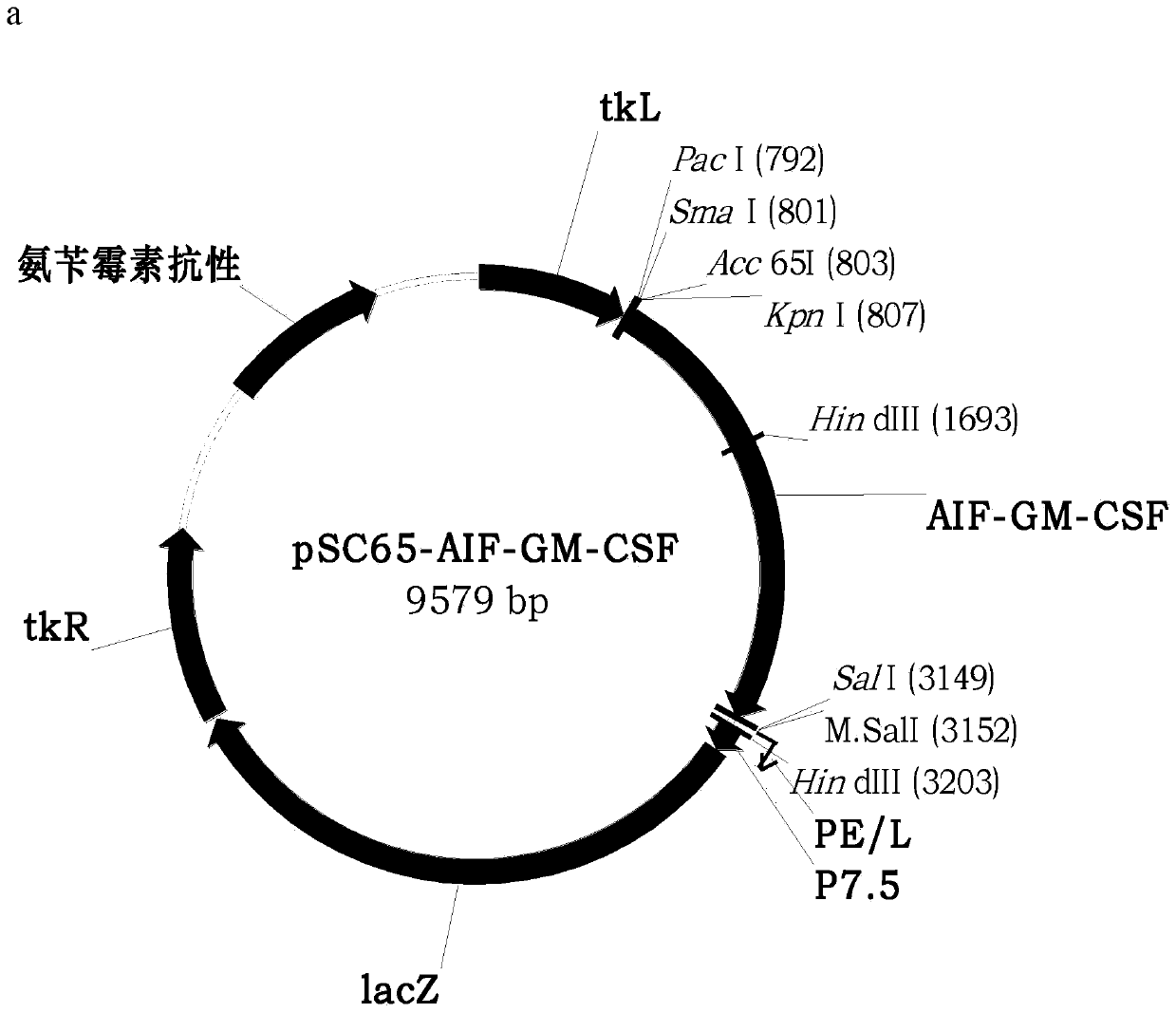
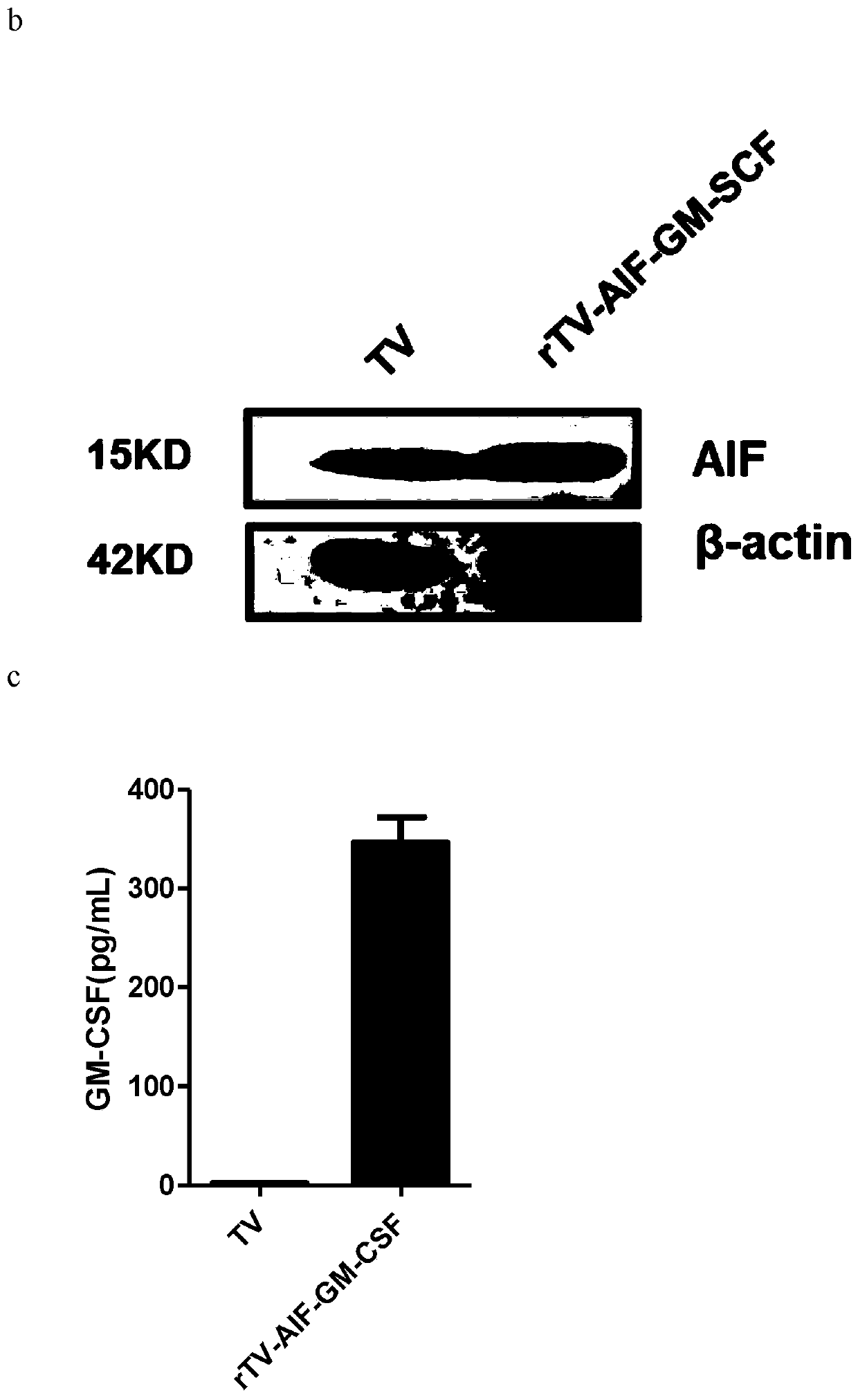
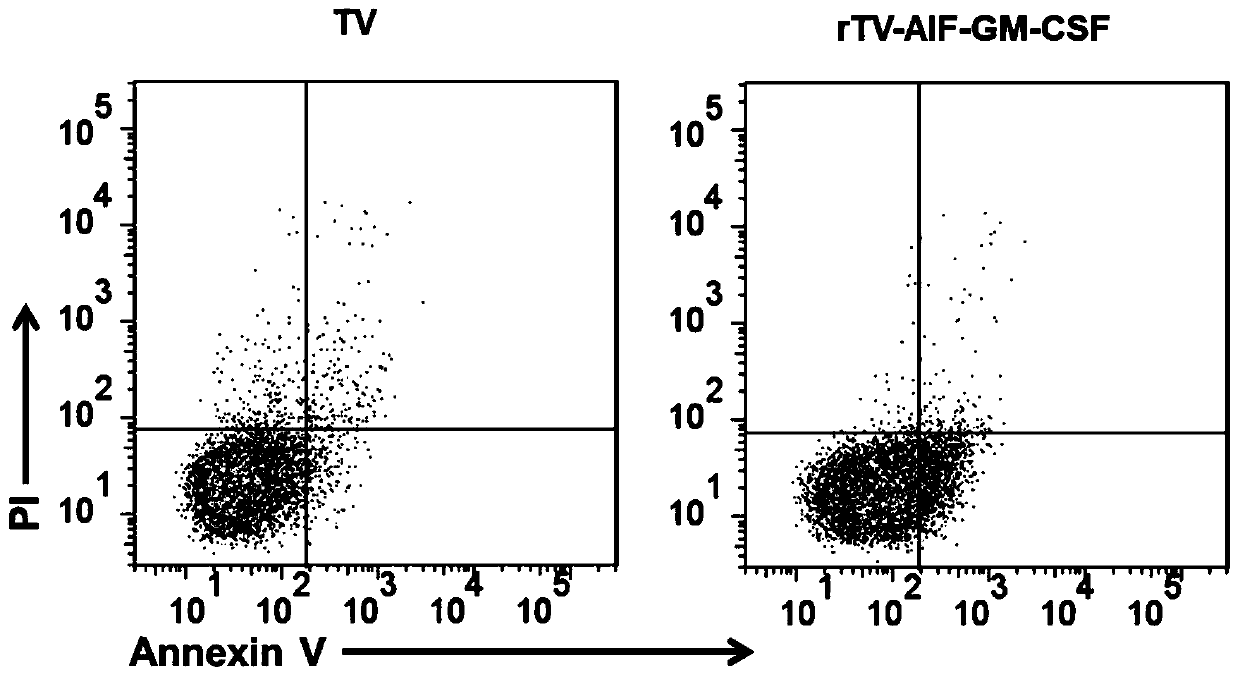
Novel oncolytic virus, as well as preparation method and application thereof
ActiveCN110499297APromote value-added differentiationIncrease lethalityMicroorganism based processesPeptidesNatural Killer Cell Inhibitory ReceptorsApoptosis
The invention discloses a novel oncolytic virus based on vaccinia virus Tiantan strain. The thymidine kinase (TK) region of the virus contains a coding sequence of AIF-GM-CSF as shown in SEQ ID NO.1.The oncolytic vaccinia virus which can efficiently expressing a human AIF-GM-CSF gene is prepared by effectively combining the tumor suppression effect of gene therapy and the oncolytic effect of viral therapy. While the oncolytic virus of the vaccinia virus Tiantan strain achieves the oncolytic effect to pyrolyze tumor cells, human AIF is massively expressed to cause apoptosis of a great number of infected tumor cells; and a great number of human GM-CSF can be expressed, and NK cells or DC cells can be recruited into the inside of a tumor to kill the tumor or effectively represent a tumor antigen, so that proliferation and differentiation of cytotoxicity t cells can be improved, and multiple anti-tumor effects can be achieved. Compared with simple gene therapy or virus therapy, the malignant tumor killing performance of the novel oncolytic virus is reinforced.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Protein carrier system for therapeutic oligonucleotides
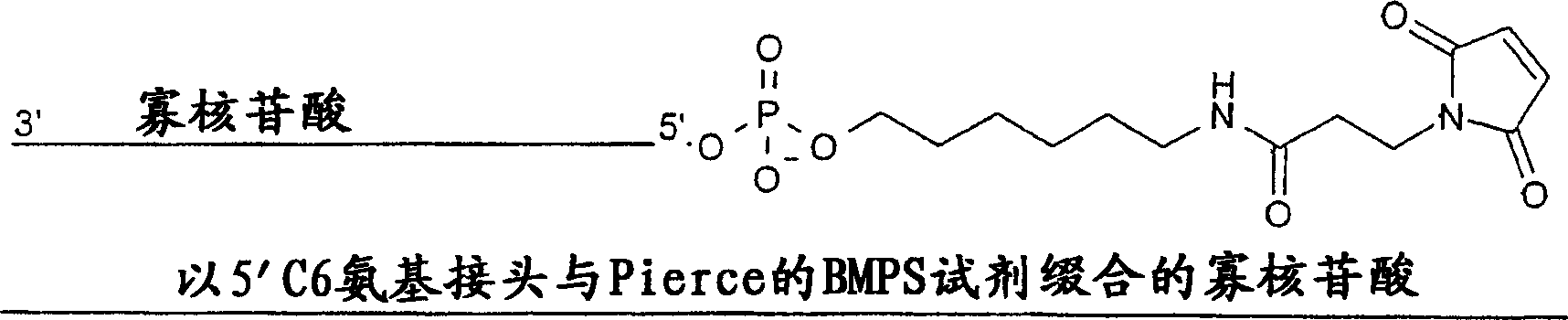
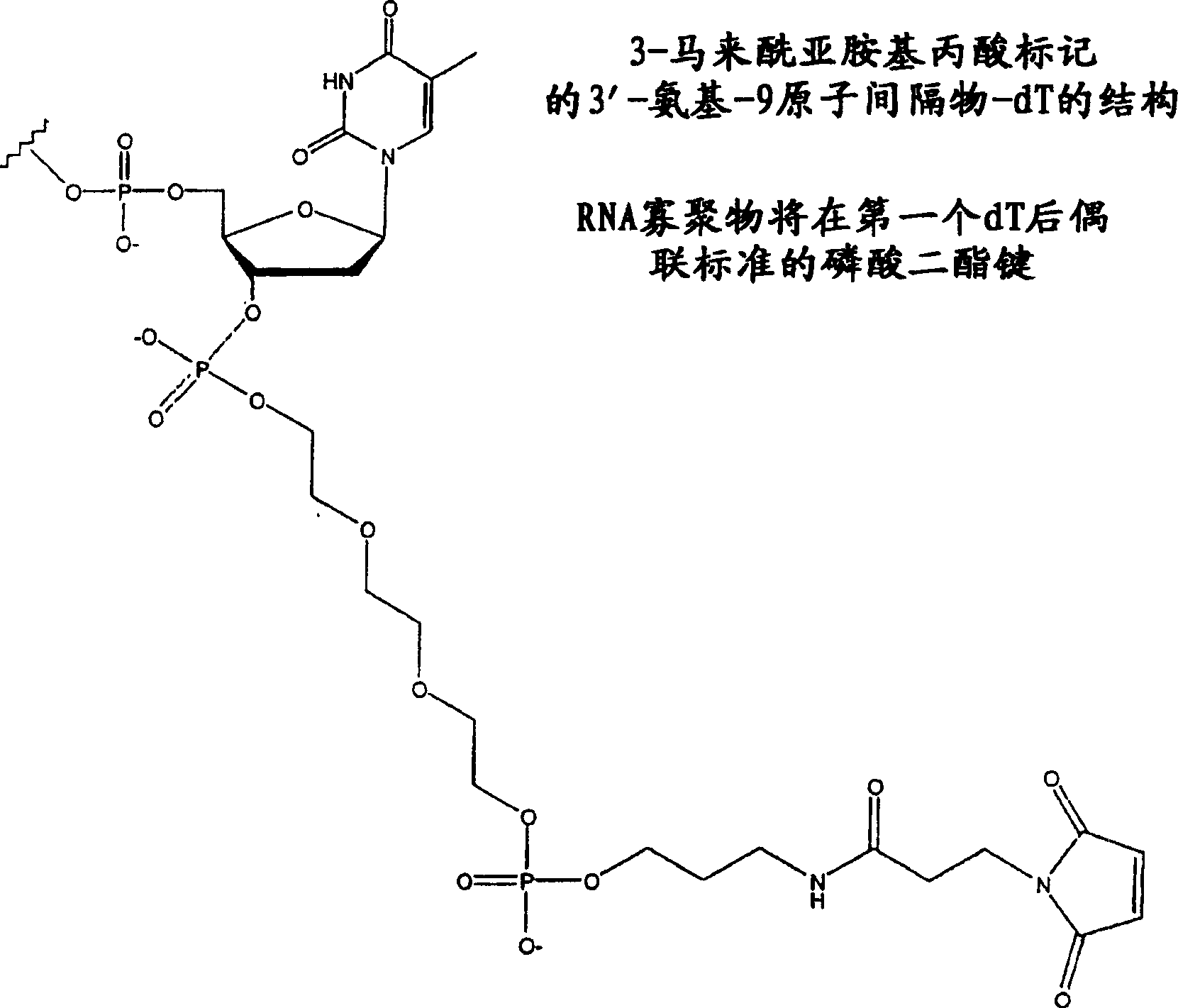
Therapeutic oligonucleotides, including antisense oligonucleotides and siRNA, are modified with reactive chemical groups linked by flexible linker molecules. Modified oligonucleotides are capable of forming covalent bonds with mobile proteins, particularly human serum albumin. The resulting complexes possess enhanced cell entry, significantly increased serum half-life, and reduced immune system stimulation, while retaining biological activity, compared to unmodified oligonucleotides. Modified oligonucleotides overcome many of the problems associated with current antisense drugs. A modified oligonucleotide of the invention is administered as a therapeutic agent and hybridizes to a complementary sequence within the targeted RNA molecule.
Owner:成都海圻生物科技有限公司 +1
Molecular programming of nanoparticle systems for an ordered and controlled sequence of events for gene-drug delivery
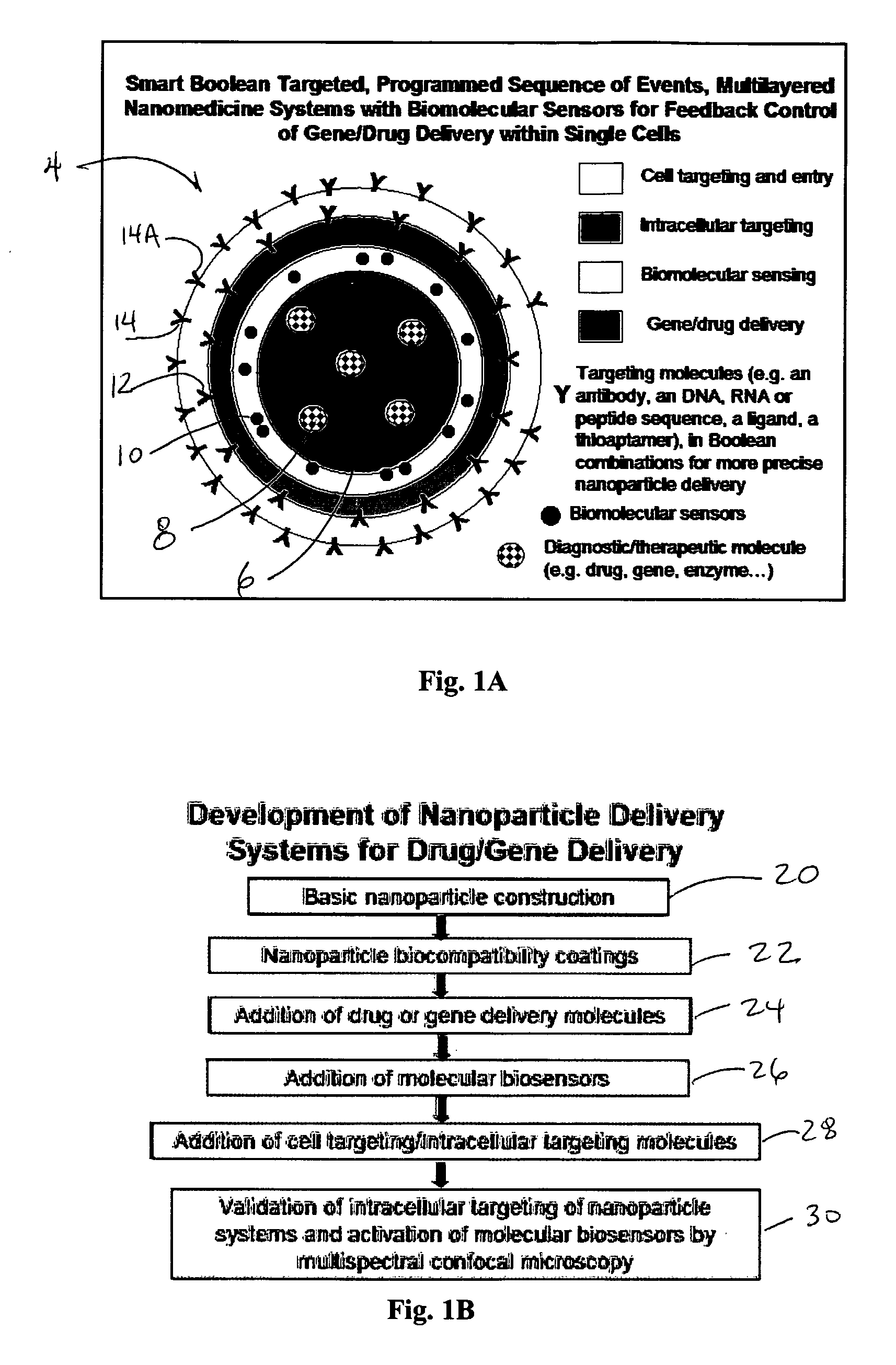
InactiveUS20070190155A1Reduce false positive targetingLower undesiredPowder deliveryMicroencapsulation basedGene deliveryMolecular programming
The disclosure provides a nanodelivery system and related process having complex, multilayered nanoparticles for sophisticated drug / gene delivery systems to intracellular portions of a cell. Outermost layers can include cell targeting and cell-entry facilitating molecules. The next layer can include intracellular targeting molecules for precise delivery of the nanoparticle complex inside the cell of interest. Molecular biosensors can be used to confirm the presence of expected molecules as a surrogate molecule for signs of infection, for activation in radiation damage, or other criteria, prior to delivery of counter-measure molecules such as drugs or gene therapy. The biosensors can also be used as a feedback control mechanism to control the proper amount of drug / gene delivery for each cell. Further, the nanodelivery system can be used to restrict any cells from encountering the drug unless that cell is specifically targeted. Successful targeting can be verified by 3D multispectral confocal microscopy.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Fluorogenic dyes
InactiveUS7026166B2Ultrasonic/sonic/infrasonic diagnosticsImmobilised enzymesFluorochrome DyeCell biology
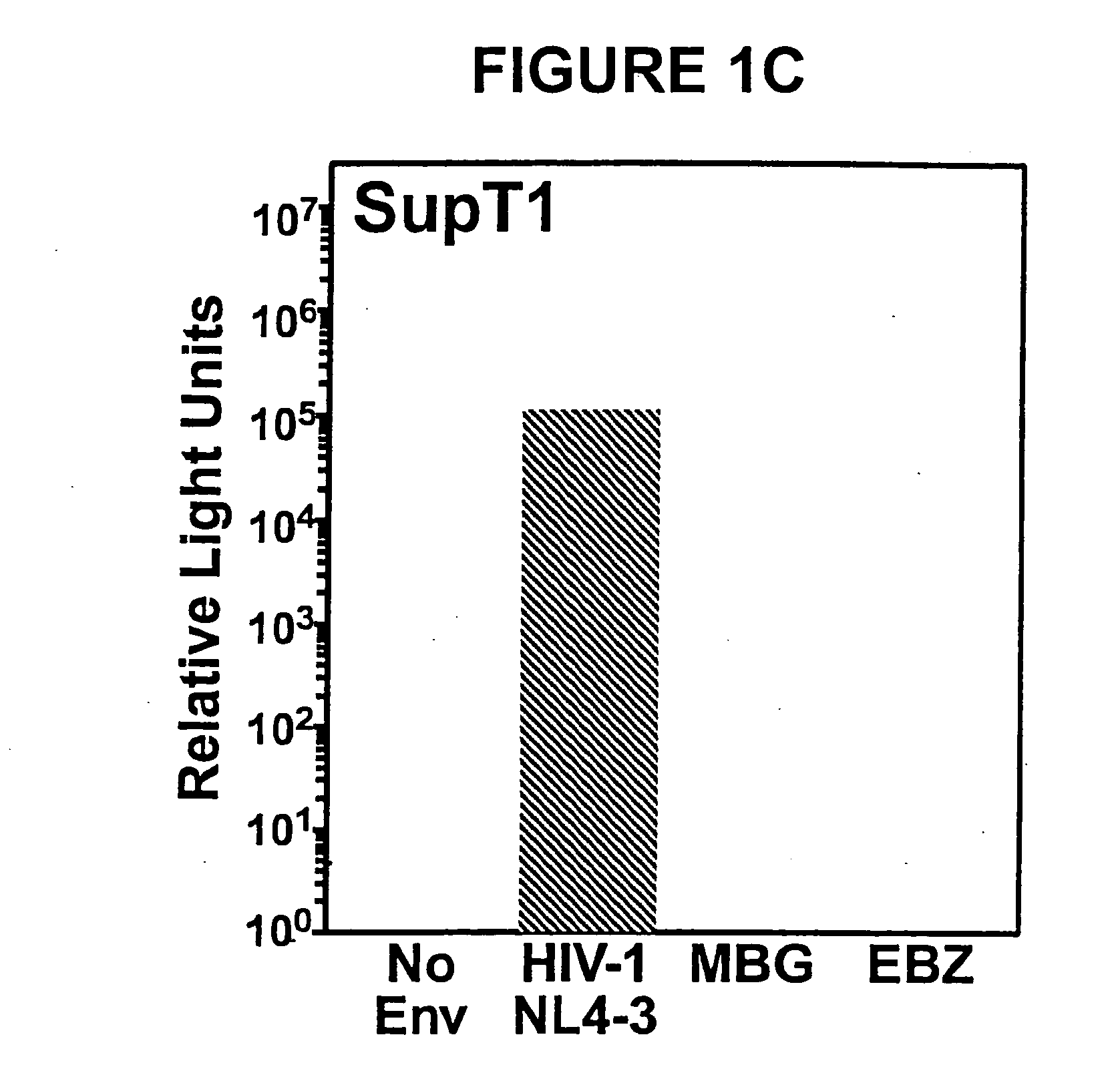
The present invention relates to the use of fluorogenic or chromogenic dyes as reporter molecules for detecting cell entry by a specific molecule.
Owner:CHIRON CORP
MicroRNA nano complex based on framework nucleic acid material as well as preparation method and application of microRNA nano complex
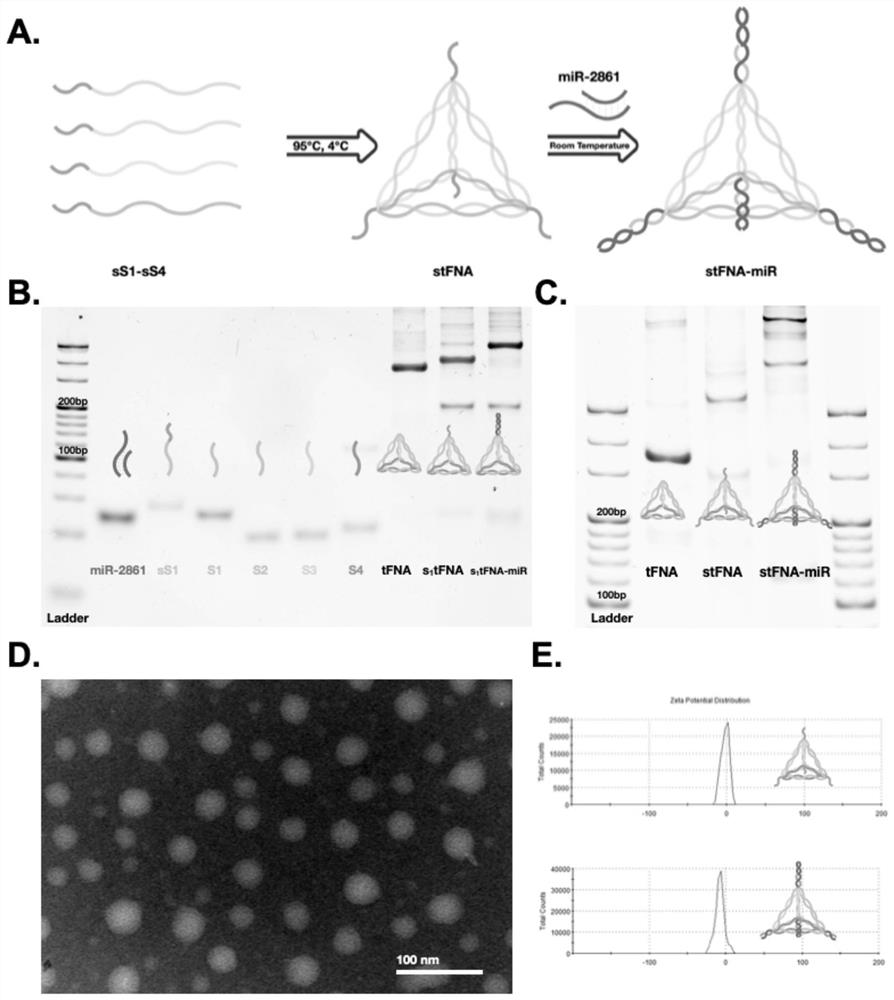
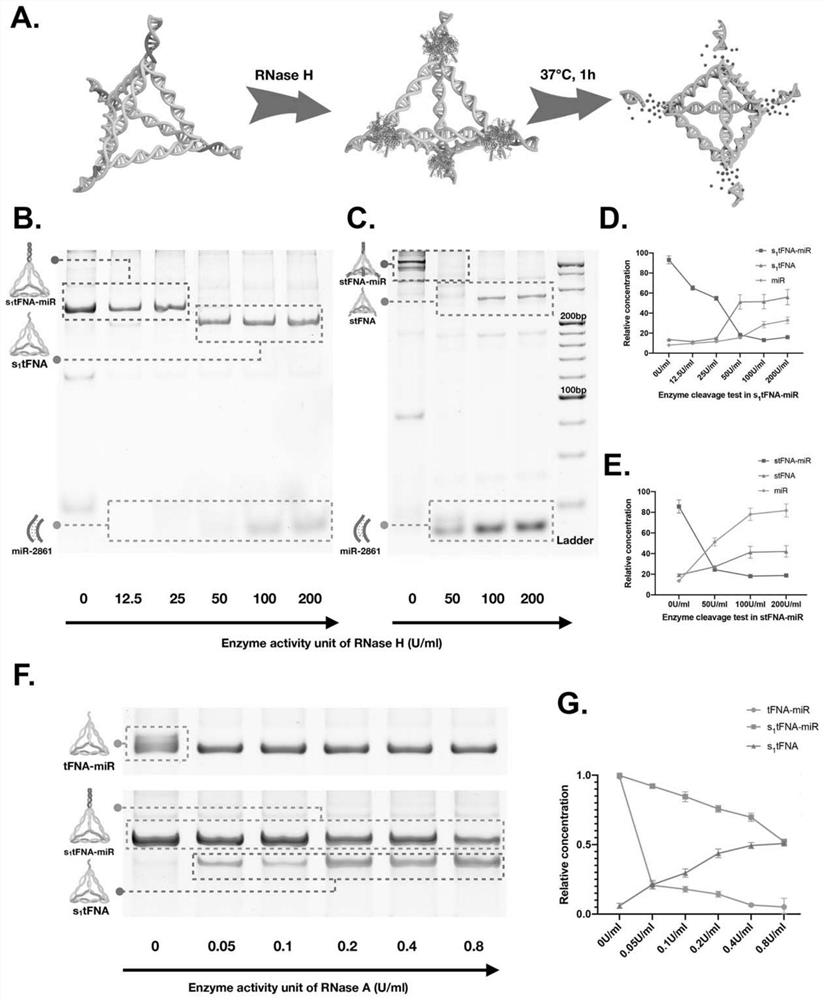
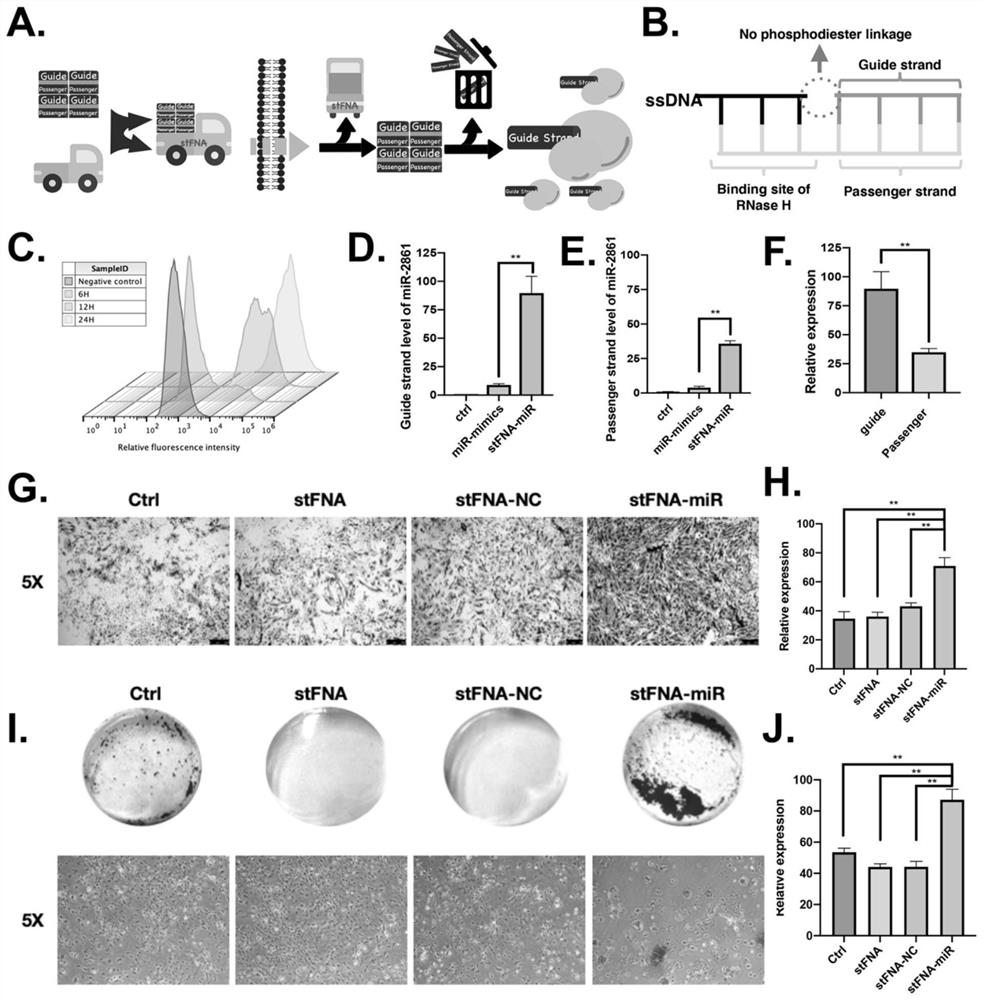
The invention provides a microRNA nano complex based on a framework nucleic acid material as well as a preparation method and application of microRNA nano complex, and belongs to the field of nucleic acid molecule medicines. The method comprises the following steps of firstly, providing a DNA tetrahedral framework nucleic acid with one or more DNA cohesive ends, and then providing a microRNA nano complex which is obtained by taking the DNA tetrahedral framework nucleic acid as a carrier and loading microRNA. According to the microRNA nano complex, the stability of the microRNA can be kept, the separation of the microRNA from a carrier DNA tetrahedron in a body can be realized, the action efficiency of the microRNA cannot be influenced, and the defects of poor stability and poor effect of a microRNA nano-composite material in the prior art are overcome. The microRNA nano composite material is high in cell entry effect, can be well ingested by BMSC, promotes osteogenic differentiation of BMSC, realizes in-vivo bone regeneration, has excellent biological activity, and is excellent in application prospect.
Owner:SICHUAN UNIV
Fluorogenic dyes
Fluorogenic or chromogenic dyes are useful as reporter molecules for detecting cell entry by a specific molecule.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
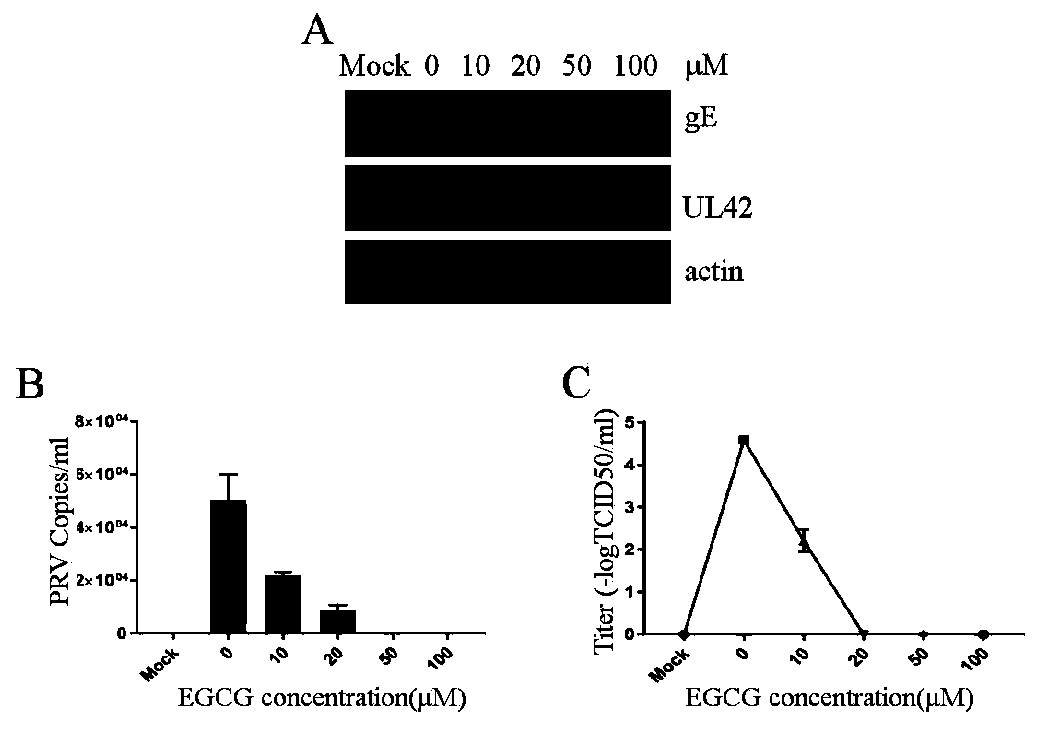
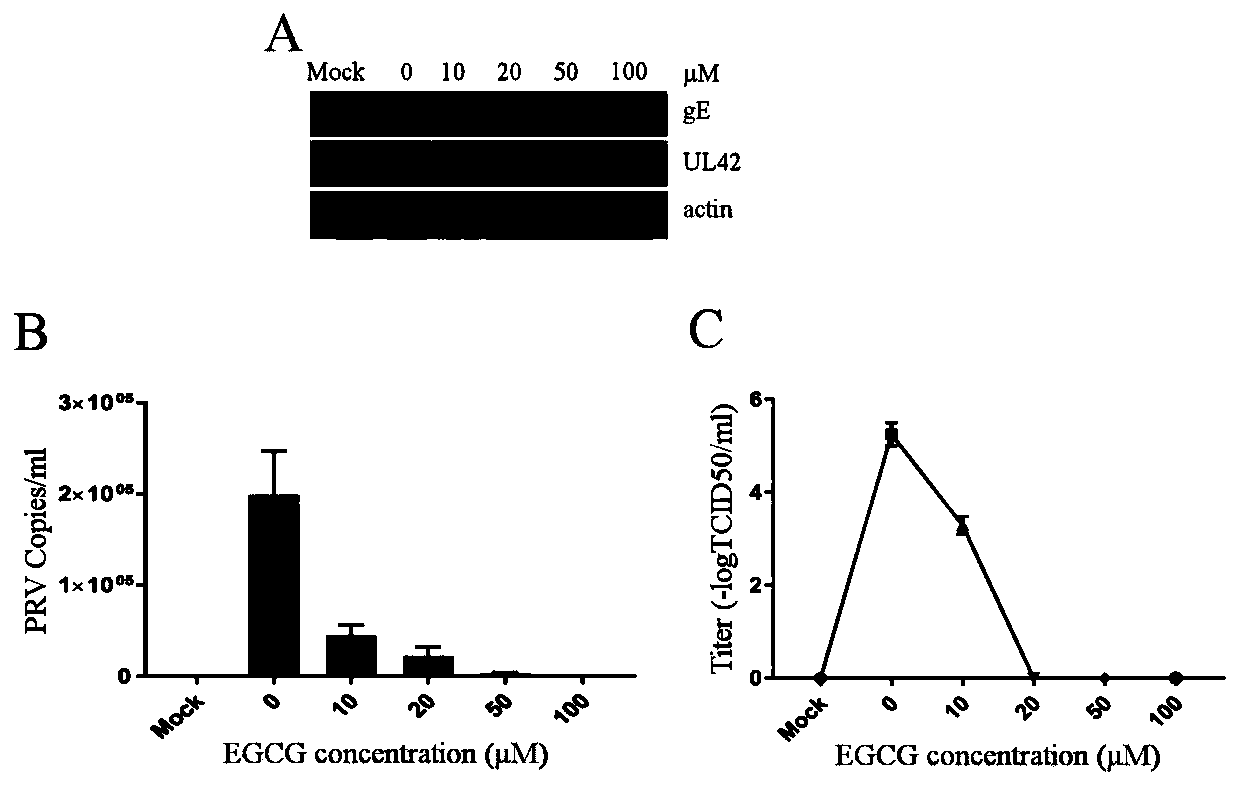
Application of EGCG in preparation of preparation for preventing and/or treating PRV infection and preparation for preventing and/or treating PRV infection
ActiveCN110731958AWide range of medicinesRaw materials are easy to getOrganic active ingredientsAntiviralsCytopathic effectRabies
The invention provides application of epigallocatechin gallate (EGCG) in preparation of a preparation for preventing and / or treating porcine pseudorabies virus infection and a preparation for preventing and / or treating porcine pseudorabies virus, and belongs to the technical field of treatment of porcine pseudorabies virus infection. The concentration of the EGCG in the medicine is 10 to 100 micromole / L. In the invention, the anti-porcine pseudorabies virus infection effect of the EGCG has is extremely remarkable: the EGCG can reduce cytopathic effect caused by porcine pseudorabies virus, theadsorption, cell entry and replication of the porcine pseudorabies virus are inhibited, the porcine pseudorabies virus can be directly killed, more importantly, the EGCG can prevent and / or treat miceinfected by the porcine pseudorabies virus, and a scientific and reliable theoretical basis is provided for clinical treatment of the porcine pseudorabies virus.
Owner:YANGZHOU UNIV
Polydopamine-doped glucan hydrogel porous scaffold as well as preparation method and application thereof
PendingCN111748120AGood biocompatibilityPromote degradationArtificial cell constructsCell culture supports/coatingCross linkerBiocompatibility
The invention relates to a polydopamine doped glucan hydrogel porous scaffold as well as a preparation method and application thereof. The method is used for cell in-vitro 3D culture and wound repair.The hydrogel scaffold is prepared by the steps as follows: preparing the hydrogel scaffold; dissolving glucan by using a sodium hydroxide solution to produce a glucan solution; adding dopamine hydrochloride into a sodium hydroxide solution for oxidative polymerization to generate a polydopamine solution; uniformly mixing the two components according to a certain ratio, and then adding a cross-linking agent ethylene glycol glycidyl ether; and placing the mixture in a 37 DEG C constant-temperature box for 12 hours to form gel. Herein, the glucan used in the invention is a high-water-solubilitynatural polysaccharide secreted by bacteria and has good biocompatibility and degradability, therefore, a large number of micropore structures are generated after gel is formed, the large specific surface area can provide a large number of loading sites for polydopamine, cells can enter the gel easily and adhere to the polydopamine, and the porous hydrogel scaffold material is excellent and has wide application prospects in wound repair and biological tissue engineering.
Owner:WENZHOU MEDICAL UNIV
Gluten-related disorders
ActiveUS20150174199A1Reduce gliadin peptide toxicityReduce cellular entryBiocideNervous disorderMetaboliteGluten-related disorders
The present invention features compositions and methods for treating gluten-related disorders. We describe compositions comprising one or more metabolites produced by Lactobacillus paracasei CBA L74, International Depository Accession Number LMG P-24778 that reduce cellular entry of gliadin peptides. The compositions may include a physiologically acceptable carrier, for example, a food product or a pharmaceutical carrier. The compositions can be administered to a subject having a gluten-related disorder, for example, celiac disease or gluten sensitivity.
Owner:H J HEINZ COMPANY BRANDS
Application of sea elastase myroilysin in preparation of acellular dermal matrix
ActiveCN103589680AEasy accessPromote proliferationMicroorganism based processesVertebrate cellsPorosityCuticle
The invention relates to application of sea elastase myroilysin in the preparation of an acellular dermal matrix, and the preparation of the acellular dermal matrix comprises the following steps: (1) performing fermentation culture of an activated myroides profundi D25 strain, centrifugating, taking a supernatant, adding ammonium sulfate, collecting a precipitation, centrifugating after redissolving and dialysis, separating and purifying by ion exchange chromatography to obtain the elastase myroilysin; (2) dissolving the elastase myroilysin in a Tris-HCl buffer solution to prepare an enzyme solution; (3) dipping fatty layer and epidermis removed pigskin in the enzyme solution for processing, and taking out for washing to prepare the pigskin acellular dermal matrix. The sea-derived elastase myroilysin has high acellular and elastase removal efficiency, is fast in speed and small in use amount; and the prepared acellular dermal matrix is large in aperture and high in porosity, facilitates cell entry and proliferation in tissue engineering, and has wide uses in the tissue engineering.
Owner:SHANDONG UNIV +1
Traditional Chinese medicine polysaccharide and preparation method and application thereof
PendingCN111544447AIncrease the number ofFunction increaseAntibacterial agentsOrganic active ingredientsBiotechnologyWhite blood cell
The invention discloses traditional Chinese medicine polysaccharide. The traditional Chinese medicine polysaccharide is prepared from the following raw materials in parts by weight: 20 to 70 parts offructus alpiniae oxyphyllae polysaccharide, 20 to 70 parts of betel nut polysaccharide and 0 to 30 parts of auxiliary materials, the traditional Chinese medicine polysaccharide can be applied to prevention and treatment on infectious diseases of live pigs, a reasonable traditional Chinese medicine formula is selected, the main component is natural south medicine polysaccharide, virus attachment cells can be directly inhibited from entering a cell and virus replication process, the plant polysaccharide serving as an interferon inducer can enhance the functions of macrophages and T cells, so that the number of E-ring forming cells is increased, cytokines are induced, production of interleukin 2 (IL-2) is promoted, endogenous interferon is produced in an animal body, and the antiviral purposeis achieved.
Owner:HAINAN JINZHU AGRI DEV CO LTD
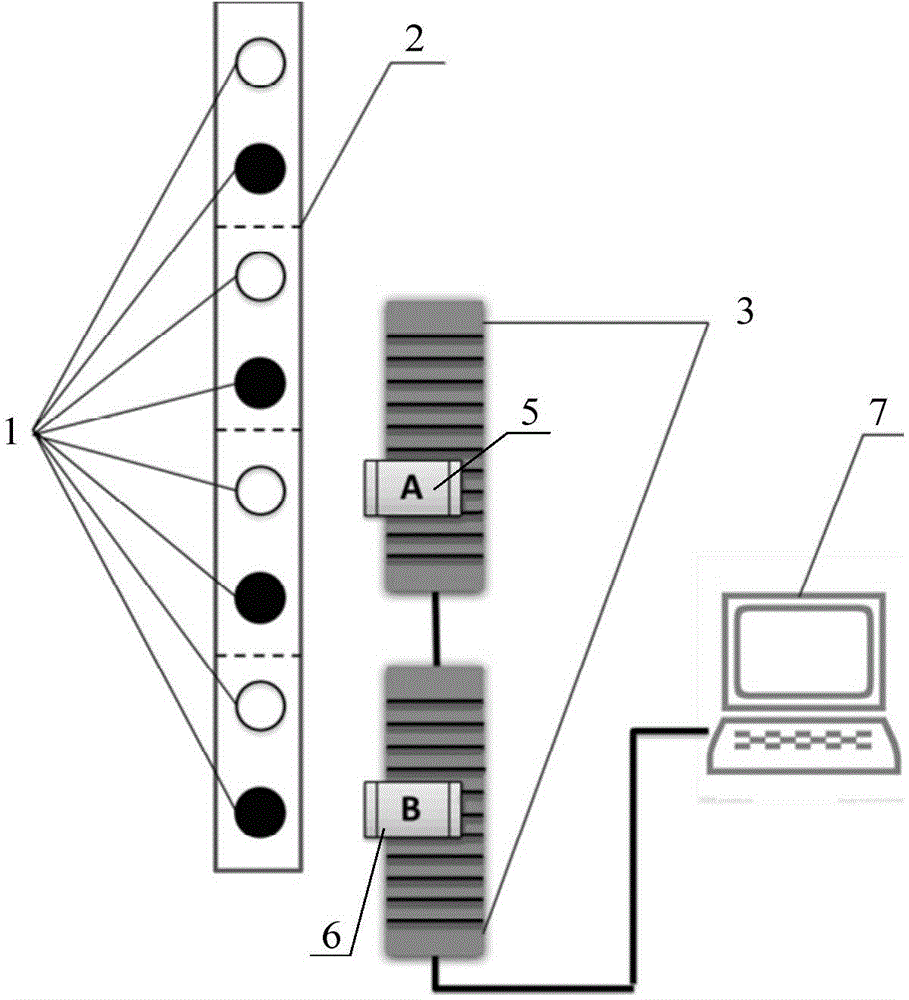
Bioanalysis chip capable of realizing cell capture and fixation
PendingCN111774110ANot easy to loseAchieve captureLaboratory glasswaresBiological testingBio moleculesCell trapping
The invention relates to the fields of micro-fluidic chips, cell operation and biological analysis, in particular to a bioanalysis chip capable of realizing cell capture and fixation. The bioanalysischip comprises a bioanalysis substrate and a micropore array chip, the micropore array chip comprises a substrate and a micropore array formed by a plurality of micropores on the substrate, and the inner diameter of micropore openings of the micropores is smaller than that of micropore cavities. According to the micropore structure, the diameter of the opening of the micropore is slightly larger than the diameter of cells, single cell capture can be achieved, the cell is not prone to loss after entering the micropore cavity, the bioanalysis chip can be used for multi-time-point detection of the same cell, and the micropore cavity can provide sufficient space and culture solution for long-time culture of various cells. The bioanalysis chip can be used for cell multi-index biomolecular analysis, cell multi-index multi-time-point dynamic biomolecular analysis and single-cell multi-index multi-time-point biomolecular analysis, such as secretory protein spectrum analysis and exosome analysis.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Cell-Penetrating Peptides Having a Central Hydrophobic Domain
ActiveUS20140342992A1Enhance cell viabilityIncrease resistanceNervous disorderPeptide/protein ingredientsArginineDelivery vehicle
The present invention discloses cell penetrating peptides (CPP or membrane translocating peptide) and their conjugates with cargo molecules. The peptides are useful as drug delivery systems, particularly as delivery vehicles for nucleotide-based theraputics, such as polynucleotides, oligonucleotides and peptide nucleic acids. A CPPs of the invention provides a balance between good cell entry efficency and low toxicity and comprises three contiguous domains: the central one being hydrophobic and the flanking ones consisting of arginine and aminohexanoic acid or beta-alanine residues. The hydrophobic domain contains a sequence selected from YQFLI, YRFLI, IQFLI and IRFLI.
Owner:UK RES & INNOVATION LTD
High-flux shooting method used for flow cytometry imaging
InactiveCN104486549AIncrease flow rateImprove shooting efficiencyTelevision system detailsColor television detailsHigh fluxShooting method
The invention belongs to the field of imaging technologies and provides a high-flux shooting method used for flow cytometry imaging. The high-flux shooting method used for flow cytometry imaging aims to solve the problems in the prior art that shooting efficiency is low, luminous flux is low and shooting missing exists. The method includes the following steps that cells move from top to bottom from a micro-pipeline; when the first cell at an even number enters a view field of a camera A, the camera A is driven by a transmission device to perform tracking shooting on the first even number; the camera A resets to an initial position through the transmission device after completing shooting, then shoots the cell at the next even number and transmits a collected image to a computer; when the first cell at an odd number enters a view field of a camera B, the camera B is driven by a transmission device to perform tracking shooting on the first odd number; the camera B resets to an initial position through the transmission device after completing shooting, then shoots the cell at the next odd number and transmits a collected image to the computer; the steps are repeated until all the cells are shot.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
Clinical-grade autogenous bronchial basal layer cell, transfusion preparation and preparation technology
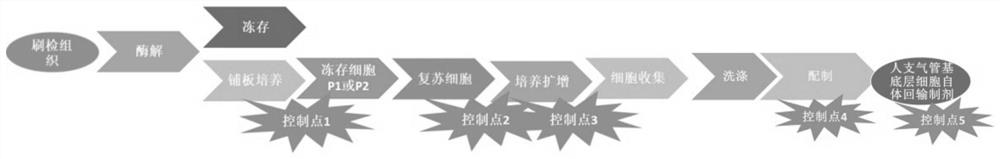
PendingCN111944737AAchieve fixEfficient repairCell dissociation methodsCulture processDigestion TreatmentBronchial epithelium
The invention discloses a clinical-grade autogenous bronchial basal layer cell, a transfusion preparation and a preparation technology in the technical filed of regeneration medicine. The preparationtechnology specifically includes the following steps: taking in-vitro active bronchial brushing tissue to perform digestive treatment, and collecting cells after digestion is finished; performing planking culture on the digested cells by using a culture plate coated with trophoblastic cells, collecting the cells and using the culture plate coated with the trophoblastic cells to perform amplification culture, and performing subculturing when the cells are grown to 50%-90% of the surface area of the culture plate; and performing digestion and collecting adherent cells when the subcultured cellsare grown to 85%-95% of the surface area of the culture dish, and then performing washing. The clinical-grade cells are firstly determined to be suitable for lung injury repair; through the selectionof proper preparation technology, the industrialized preparation of the cells can be realized, and bronchial basal layer cells that meet the quantity and quality of clinical cell treatment can be obtained within a short time; and the bronchial basal layer cells prepared by the method can stably differentiate when entering the focus, so that the obvious repairing of the lungs can be realized.
Owner:REGEND THERAPEUTICS CO LTD
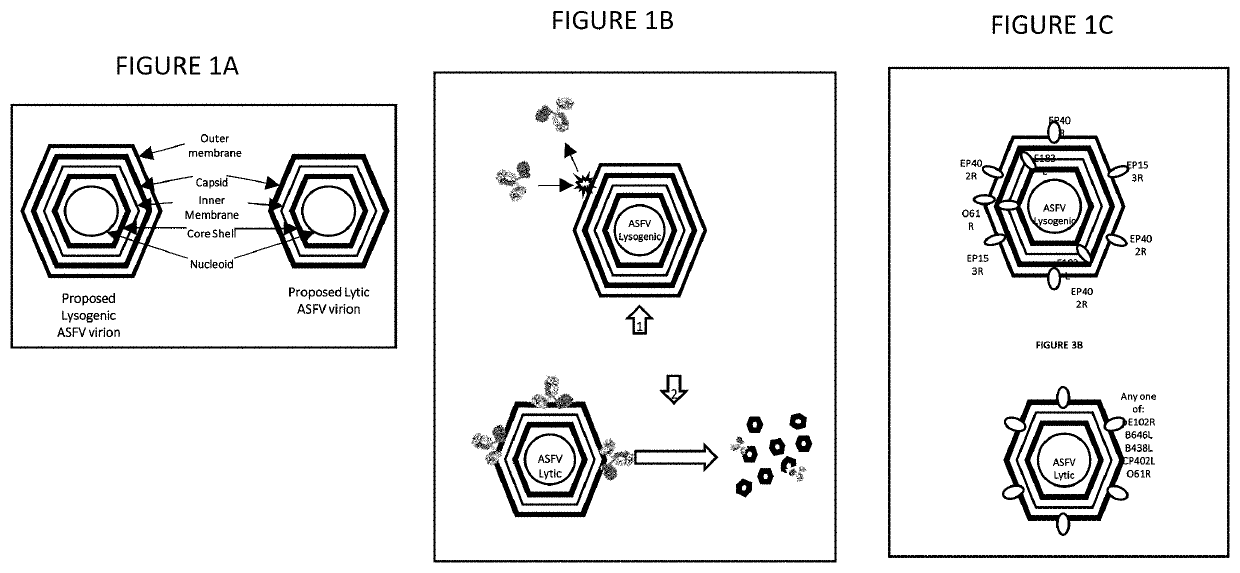
Methods of blocking asfv infection through interruption of cellular and viral receptor interactions
PendingUS20220241391A1Peptide/protein ingredientsViral antigen ingredientsViral ReceptorTreated animal
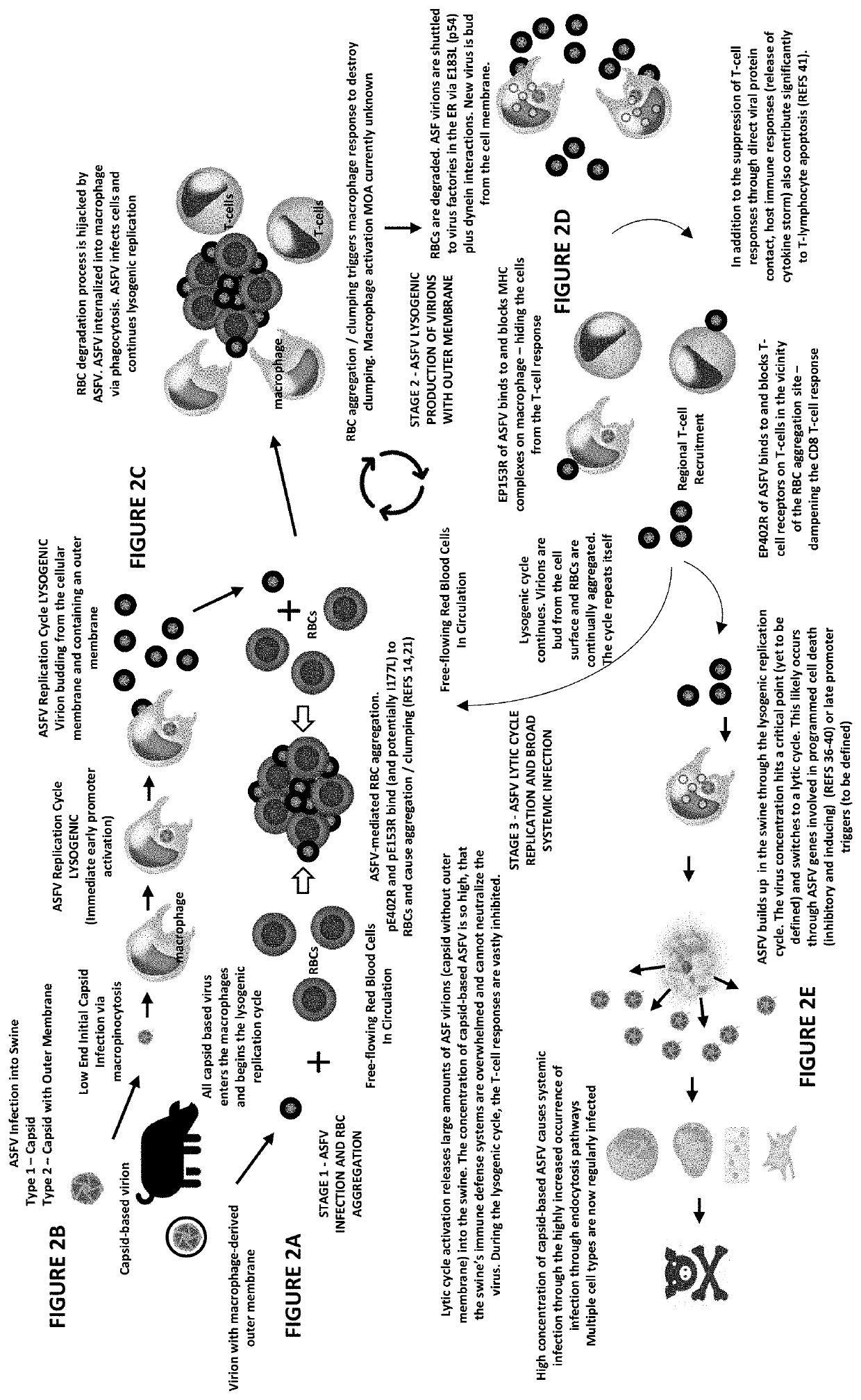
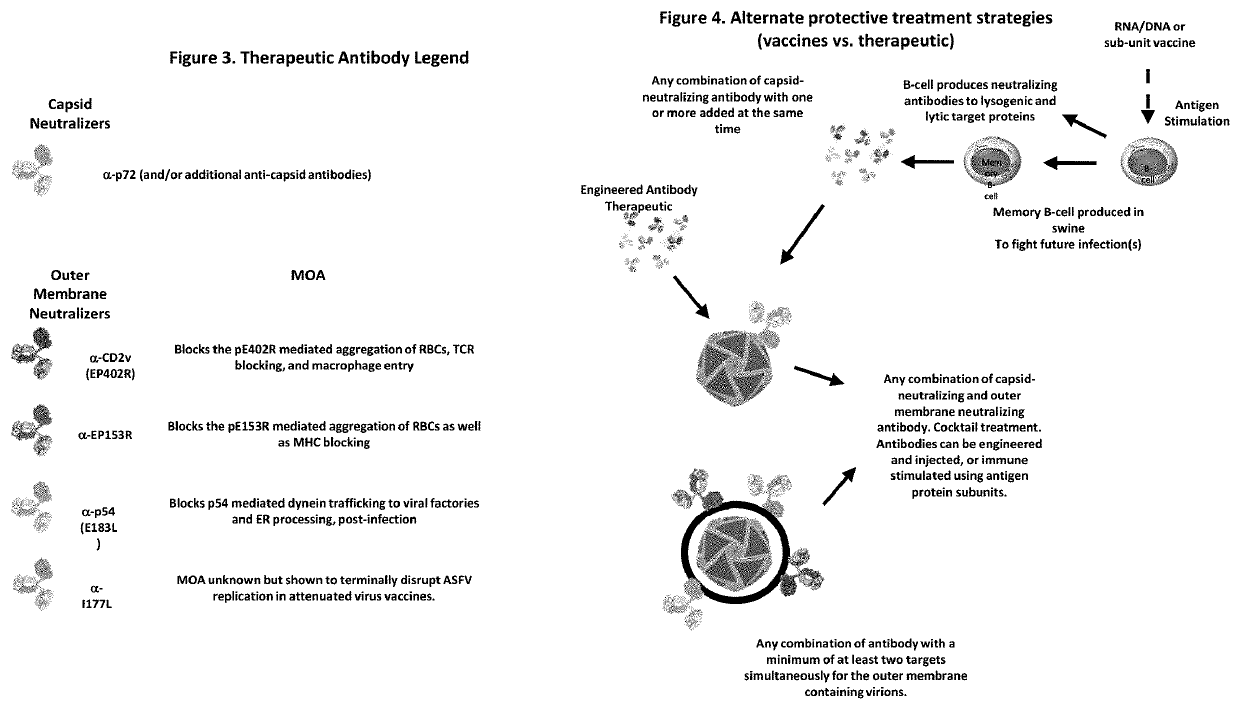
A method of preventing and treating viral infections in animals (and preferably ASFV in porcine), by inhibiting viral ligand interactions with critical cellular receptors that are involved either directly (endocytosis and / or macropinocytosis) or indirectly (phagocytosis of RBCs that have been aggregated by viral interactions) with cellular entry in an animal, and preventing and treating the viral infection in the animal. A method of treating a viral infection in an individual with a virus that is both lysogenic and lytic. A composition for treating a viral infection in an individual with a virus that is both lysogenic and lytic. A vaccine for preventing viral infection, including whole and / or partial domains of proteins of both a lysogenic and lytic phase of a virus.
Owner:CHEN DALU +1
Methods and compositions for use in the treatment of filovirus mediated disease conditions
Methods and compositions are provided for at least slowing the progression of a filovirus mediated disease condition in a host. In the subject methods, an effective amount of an agent that at least reduces the amount of folate receptor mediated filovirus cell entry is administered to the host. The subject methods find use in both the prevention and treatment of filovirus associated disease conditions, including Marburg and Ebola-Zaire virus mediated disease conditions.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Gluten-related disorders
ActiveUS10245300B2Reduce gliadin peptide toxicityReduce cellular entryNervous disorderPeptide/protein ingredientsBiotechnologyMetabolite
The present invention features compositions and methods for treating gluten-related disorders. We describe compositions comprising one or more metabolites produced by Lactobacillus paracasei CBA L74, International Depository Accession Number LMG P-24778 that reduce cellular entry of gliadin peptides. The compositions may include a physiologically acceptable carrier, for example, a food product or a pharmaceutical carrier. The compositions can be administered to a subject having a gluten-related disorder, for example, celiac disease or gluten sensitivity.
Owner:H J HEINZ COMPANY BRANDS
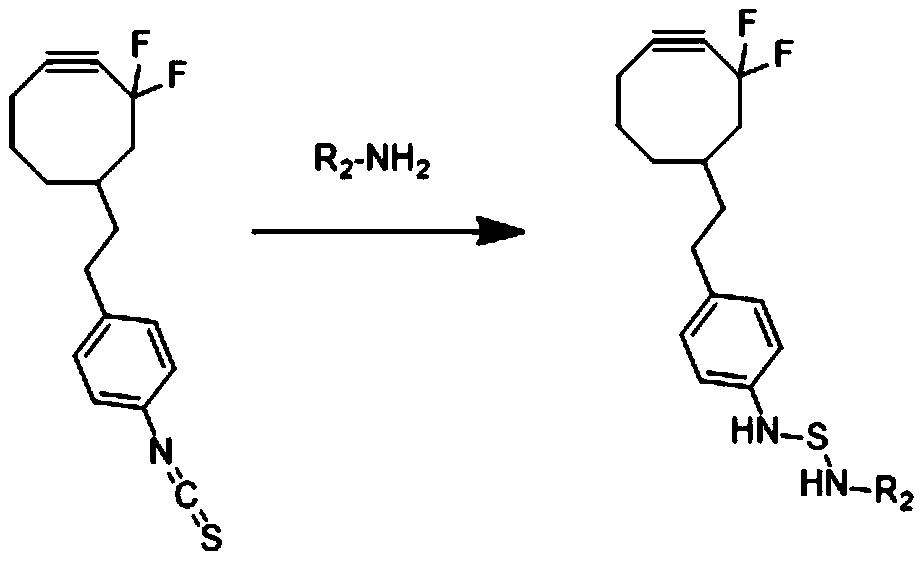
CAR-T living body tracing method
ActiveCN110917365AWon't hurtAvoid damageRadioactive preparation carriersBlood/immune system cellsHuman bodyMolecular binding
The invention relates to the technical field of living body tracing, in particular to a CAR-T living body tracing method, and aims to solve the problem that in the prior art, radioactive nuclein enters living bodies (animals or human bodies) along with CAR-T cells to damage the CAR-T cells and the living bodies. The method is characterized by comprising the following steps of S1, combining pre-landed molecule A with amino on the surfaces of the CAR-T cells to obtain the pre-landed CAR-T cells having A groups, wherein R2-NH2 is the CAR-T cells, and -NH2 is the amino groups of the surfaces of the CAR-T cells; S2, combining 18F with homing molecules B, to obtain R1-N3 of 18F; S3, injecting the pre-landed CAR-T cells obtained in S1 into the living bodies, during required imaging time points, injecting the R1-N3 into the bodies, and combining the R1-N3 with the pre-landed CAR-T; and S4, through PET / CT scanning, observing the distribution condition of the CAR-T cells in the living bodies. According to the CAR-T living body tracing method, the operation safety of experimenters is improved, and damage of radioactivity to the CAR-T cells and the living bodies can be avoided.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com