Protein carrier system for therapeutic oligonucleotides
An oligonucleotide, therapeutic technology, applied in the field of therapeutic oligonucleotides, can solve the problems of low intracellular delivery efficiency, immune system stimulation, unsatisfactory half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Embodiment 1: antisense oligonucleotide synthesis
[0156] Specific antisense oligonucleotides MB003 and MB006 were synthesized with a phosphorothioate backbone by Trilink Biotechnology (San Diego, CA). ASO MB003 and MB006 target the mRNA transcript of the human bcl-xl gene (see Simoes-Wust et al., Int. J. Cancer, 87:582-590, 2000; US Patent No. 6,214,986). Overexpression of the human bcl-xl gene has been found to be associated with many cancer cells. Therefore, treatment with an ASO specific for the human bcl-xl gene should reduce or eliminate the proliferation or growth of a variety of cancer cells. The serials of the MB003 and MB006 ASOs are listed below:
[0157] MB-003: 5'-AAAGTATCCCAGCCGCCGTT-3' (SEQ ID NO: 21)
[0158] MB-006: 5'-TCCCGGTTGCTCTGAGACAT-3' (SEQ ID NO: 22)
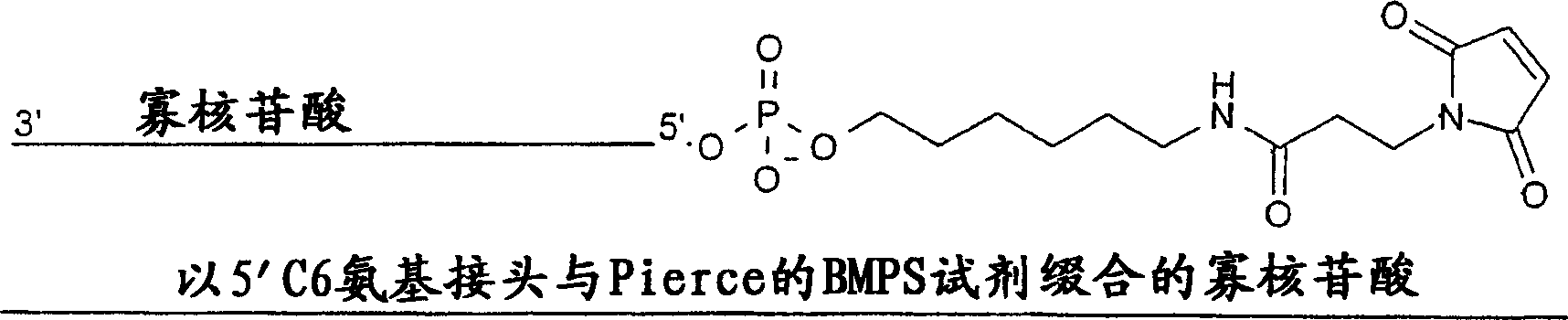
[0159] Additionally, synthesized as figure 1 Two ASOs containing a 5'-(BMPS)(C6NH) linker and a maleimide reactive group are shown. These two ASOs, designated MB-003M and MB-006M, have a 5'...
Embodiment 2
[0170] Example 2: siRNA conjugates of the invention
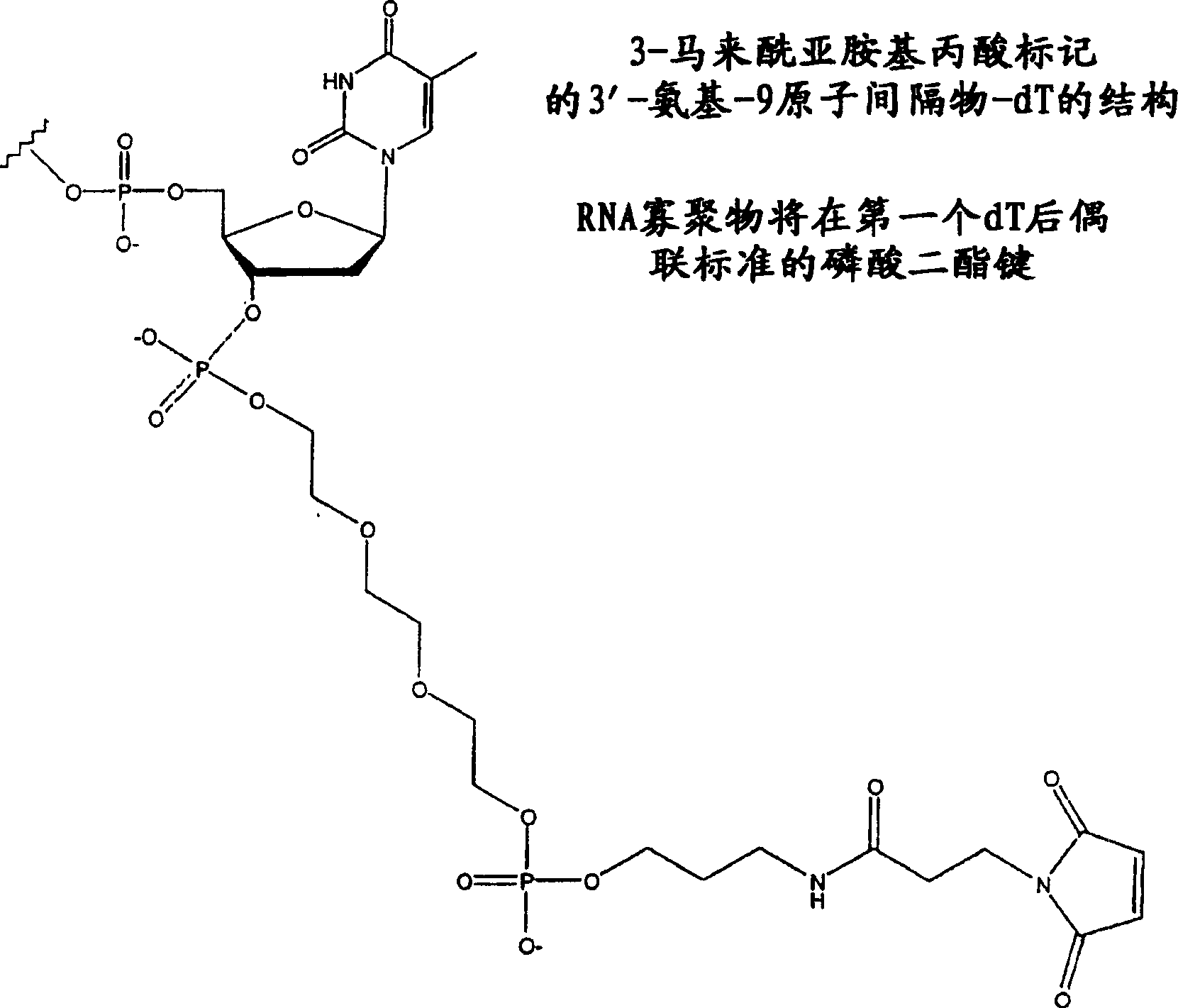
[0171] Therapeutic oligonucleotides of the invention composed of modified siRNAs with reactive groups were synthesized. First, the "sense" strand of the duplex RNA is synthesized using known techniques. Add N3_9S-MPA linker at the 3′ end ( figure 2 ) to synthesize the "sense" strand. The synthetic strands with linkers are recovered and purified. The other RNA strand complementary to the sense strand is synthesized, recovered, and purified using known techniques.
[0172] After purification, the "sense" RNA strand is annealed together with the complementary RNA strand to generate siRNA duplex molecules. After annealing, any other purification steps deemed necessary are performed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com