Clinical-grade autogenous bronchial basal layer cell, transfusion preparation and preparation technology
A preparation process and bronchial technology, applied in the field of regenerative medicine, can solve the problems of lack of bronchial basal layer cells, organs or tissue sources, increased risk of opportunistic infections, etc., to achieve safety and effectiveness, low endotoxin content , the effect of low streptomycin residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
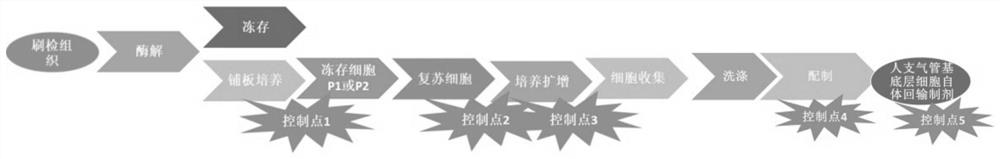
[0077] This embodiment provides a preparation process of clinical-grade autologous bronchial basal layer cells, which specifically includes the following steps:
[0078] The isolated active bronchi were collected for brush examination for later use. In this embodiment, the tissues located in two different parts of the bronchi were selected. The reason why two or more parts are selected is to ensure the success rate of separation.
[0079] Take the tissue digestion solution and stop solution for later use; 99v% of the tissue digestion solution is DMEM / F12, the rest is 1-20ng / mL DNase, 0.1-4mg / mL protease XIV and 10-200ng / mL trypsin; stop 90v% of the liquid is DMEM, and 10v% is FBS.
[0080] The above-mentioned brushed tissue is digested with the tissue digestion solution, and the digested tissue is terminated with the stop solution, and then the cells are collected.
[0081] Take the medium for later use, in which 225mL DMEM, 225mL F12, 20-70mL FBS, 0.2-2mM L-glutamine (L-glu...
Embodiment 2
[0092] This embodiment provides a preparation process of clinical-grade autologous bronchial basal layer cells, which specifically includes the following steps:
[0093] The isolated active bronchi were collected for brush examination for later use. In this embodiment, the tissues located in two different parts of the bronchi were selected. The reason why two or more parts are selected is to ensure the success rate of separation.
[0094] Take the tissue digestion solution and stop solution for later use; 99v% of the tissue digestion solution is DMEM / F12, the rest is 1-20ng / mL DNase, 0.1-4mg / mL protease XIV and 10-200ng / mL trypsin; stop 90v% of the liquid is DMEM, and 10v% is FBS.
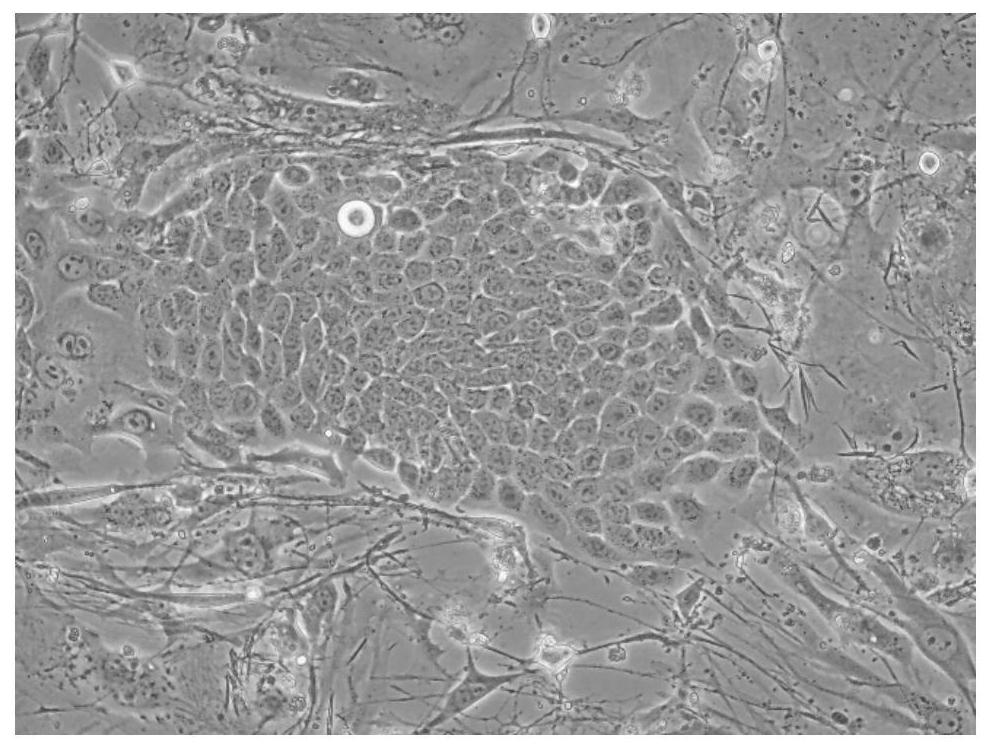
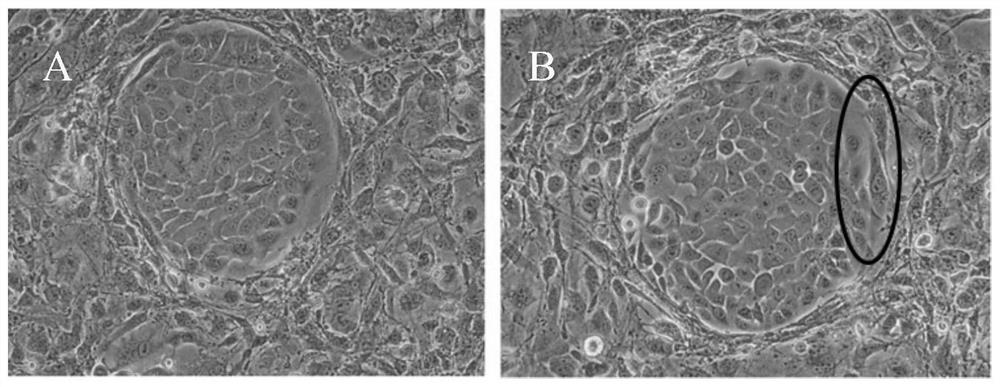
[0095] The above-mentioned brushed tissue is digested with the tissue digestion solution, and the digested tissue is terminated with the stop solution, and then the cells are collected. The cells after enzymatic hydrolysis were tested for bacteria and mycoplasma, and some of them were frozen.
...
Embodiment 3
[0109] This embodiment provides a preparation process of clinical-grade autologous bronchial basal layer cells, which specifically includes the following steps:
[0110] The isolated active bronchi were collected for brush examination for later use. In this embodiment, the tissues located in two different parts of the bronchi were selected. The reason why two or more parts are selected is to ensure the success rate of separation.
[0111] Take the tissue digestion solution and stop solution for later use; 99v% of the tissue digestion solution is DMEM / F12, the rest is 1-20ng / mL DNase, 0.1-4mg / mL protease XIV and 10-200ng / mL trypsin; stop 90v% of the liquid is DMEM, and 10v% is FBS.
[0112] The above-mentioned brushed tissue is digested with the tissue digestion solution, and the digested tissue is terminated with the stop solution, and then the cells are collected. The cells after enzymatic hydrolysis were tested for bacteria and mycoplasma, and some of them were frozen.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com