Vorinostat derivative based on lithium hydroxide, and preparation method and application thereof
A technology of vorinostat and lithium hydroxide, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of inability to achieve oral administration, achieve side effects and inability to inject drug administration, steps Simple and effective cost control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
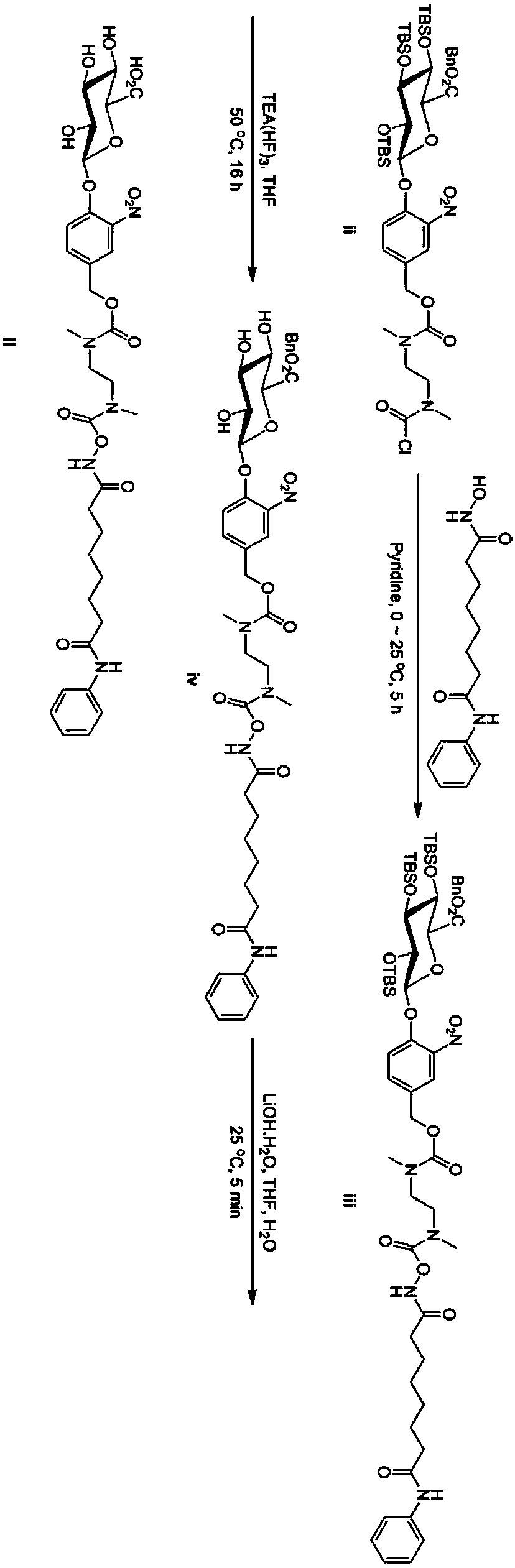
[0046] And the preparation method of vorinostat derivative of the present invention can refer to as follows:
[0047] Will Condensation reaction with vorinostat, then removal of the protecting group, and purification to obtain the vorinostat derivative;
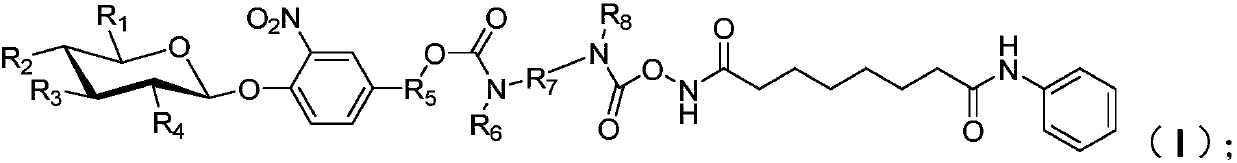
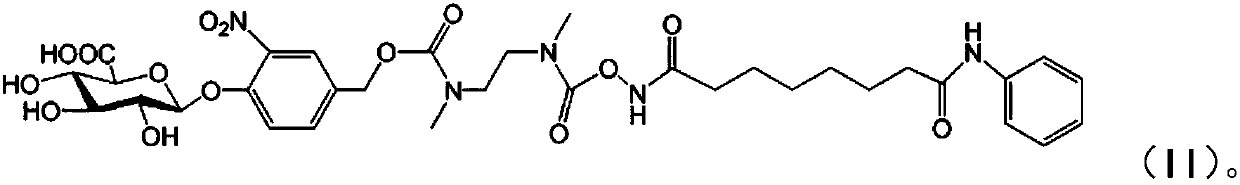
[0048] In formula (i), R 9 -R 12 are independently hydrogen, protected hydroxyl, or protected carboxyl, wherein, R 9 -R 12 There is at least one protected hydroxyl group or protected carboxyl group; preferably, in formula (i), R 9 -R 12 are independently protected hydroxyl or protected carboxyl; more preferably, in formula (i), R 9 -R 12 There is at least one protected hydroxyl group and one protected carboxyl group in, for example, R 9 is a protected carboxyl group, R 10 -R 12 is a protected hydroxyl group; alternatively, R 12 is a protected carboxyl group, R 9 -R 11 is a protected hydroxyl group; or, it can be R 9 is a protected hydroxyl group, R 10 -R 12 is a protected carboxyl group; or, R 12 is a protec...
Embodiment 1
[0072] Example 1 O-[N N-dimethyl-N-4-(2,3,4-tri-O-tert-butyldimethylsilyl-6-benzyl-β-D-glucopyranose Preparation of acid-1-yl)-3-nitrobenzyloxycarbonylethylenediamine]-formyl-vorinostat (iii)
[0073] Dissolve 913 mg (3.46 mmol) of vorinostat in 20 mL of pyridine, and cool at 0° C. for 5 minutes. At 0°C, 3 g (3.14 mmol) of compound (ii) was added to the above solution to form a red suspension. The suspension was warmed up to 25 °C, kept stirring for 5 hours, and then 100 mL of water was added to quench the reaction. The reaction solution was extracted with ethyl acetate (50mL×3 times), the combined organic layers were sequentially extracted with 0.5M HCl (30mL×4 times), saturated brine (30mL×2 times), dried over anhydrous sodium sulfate, and evaporated by rotary evaporation The mixture was concentrated and separated by preparative chromatography (dichloromethane:methanol=50:1) to obtain 3.4g of intermediate compound (iii) with a yield of 92%.
[0074] Characterization of th...
Embodiment 2
[0075] Example 2 O-[N N-dimethyl-N-4-(6-benzyl-β-D-pyranoglucuron-1-yl)-3-nitrobenzyloxycarbonylethylenediamine] - Preparation of formyl-vorinostat (iv)
[0076] Dissolve 3.4g (2.87mmol) of intermediate (iii) in 18mL of tetrahydrofuran, add 4.63g (28.7mmol) of triethylamine trihydrofluoride at 25°C, heat to 50°C, and keep the reaction for 16 hours. After the reaction is complete, the reaction solution is concentrated with a rotary evaporator, 50 mL of water is added to the concentrated solution, extracted with ethyl acetate / acetonitrile (3 / 1) (20 mL×4 times), the organic layers are combined, washed twice with saturated saline, and anhydrous sulfuric acid Sodium-dried, filtered, the filtrate was concentrated with a rotary evaporator, and separated by preparative chromatography (dichloromethane:methanol=50:1) to obtain 35.0 g of a crude product, namely the intermediate compound (iv). The yield was 74%. directly into the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com