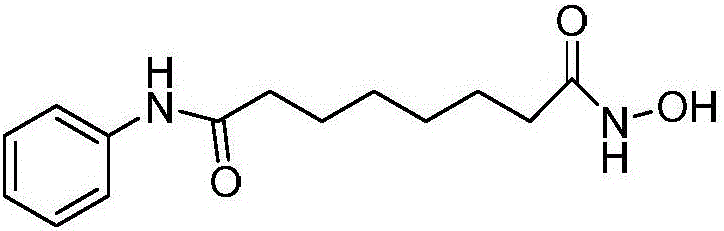
Method for preparing anticarcinogen vorinostat
A technology of vorinostat and anticancer drugs, which is applied in the field of drug synthesis, can solve the problems of low product yield and cumbersome steps, and achieve the effects of good selectivity, simple operation steps and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for preparing anticancer drug vorinostat, comprising the following steps:
[0025] 1) 15.6g (100mmol) of suberic anhydride, CuI3.8g (20mmol) and 8.8g (95mmol) of aniline were carried out in water and 1,4-dioxane (50ml mixed solvent) for contact reaction, after the reaction ended, Filtrate, adjust the pH of the filtrate to 6, filter with suction, wash the obtained filter cake with water, and dry to obtain 23.3 g of suberoyl anilide, with a yield of 93.6% and a purity of 97.25%. The temperature of the contact reaction is 8°C , the volume ratio of water and 1,4-dioxane is 1:8;
[0026] 2) Dissolve suberoyl anilide in methanol, add cation exchange resin 5.8g and ZnCl 2 1.3g (9.4mmol), heated at 50°C for 3 hours, concentrated, poured into water, extracted with ethyl acetate, concentrated, washed with petroleum ether, dried to obtain 23.4g of methyl octanoanilide, yield 95.1%, purity 98.70 %;
[0027] 3) Stir and react 6.5g (100mmol) of hydroxylamine hydrochloride...
Embodiment 2
[0029] A method for preparing anticancer drug vorinostat, comprising the following steps:
[0030] 1) Suberic anhydride 15.6g (100mmol), CuI7.6g (40mmol) and aniline 9.3g (100mmol) are carried out contact reaction in water and 1,4-dioxane (50ml mixed solvent), after reaction finishes, Filtrate, adjust the pH of the filtrate to 5, filter with suction, wash the obtained filter cake with water, and dry to obtain 22.8 g of suberoylanilide, with a yield of 91.7% and a purity of 99.82%. The temperature of the contact reaction is 5°C , the volume ratio of water and 1,4-dioxane is 1:5;
[0031] 2) Dissolve suberic acid monoanilide in methanol, add cation exchange resin 6.8g and ZnCl 2 1.9g (13.7mmol), heated at 50°C for 3 hours, concentrated, poured into water, extracted with ethyl acetate, concentrated, washed with petroleum ether, and dried to obtain 21.9g of methyl octanoanilide, with a yield of 90.6% and a purity of 97.12 %;
[0032] 3) Stir and react 6.5g (100mmol) of hydroxyl...
Embodiment 3
[0034] A method for preparing anticancer drug vorinostat, comprising the following steps:
[0035] 1) Suberic anhydride 15.6g (100mmol), CuI5.7g (30mmol) and aniline 8.4g (90mmol) were carried out in water and 1,4-dioxane (60ml mixed solvent) for contact reaction, after the reaction ended, Filtrate, adjust the pH of the filtrate to 6, filter with suction, wash the obtained filter cake with water, and dry to obtain 22.7 g of suberoyl monoanilide, with a yield of 91.0% and a purity of 95.96%. The temperature of the contact reaction is 10°C , the volume ratio of water and 1,4-dioxane is 1:10;
[0036] 2) Dissolve suberoyl anilide in methanol, add cation exchange resin 11.3g and ZnCl 2 1.8g (13.6mmol), heated at 55°C for 3 hours, concentrated, poured into water, extracted with ethyl acetate, concentrated, washed with petroleum ether, dried to obtain 21.7g of methyl octanoanilide, yield 90.8%, purity 96.43 %;
[0037] 3) Stir and react 6.5g (100mmol) of hydroxylamine hydrochlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com