Recombinant engineering bacteria expressing highly recombinant hgh and its construction method and application
A technology of recombinant engineering bacteria and expression vector, applied in the field of genetic engineering, can solve the problems of low activity and low purity of hGH, and achieve the effect of improving protein products, high product activity, and improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
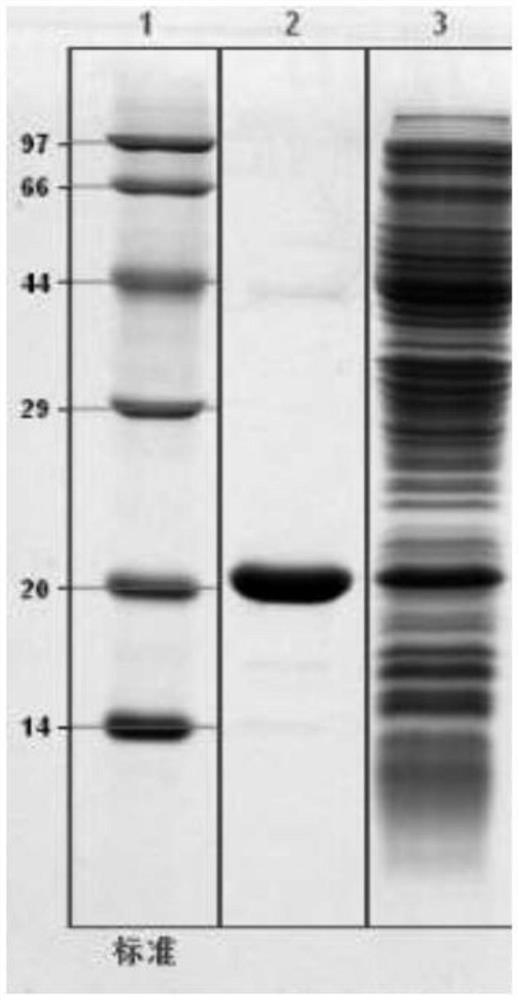
Examples
Embodiment 1
[0040] 1. Optimize the acquisition of genes
[0041] According to the known natural amino acid sequence of hGH, according to the codon preference of Escherichia coli and considering the elimination of hairpin structure and other secondary structures that are not conducive to expression, the coding sequence of hGH protein is optimized, as shown in SEQ ID NO: 1 DNA sequence.
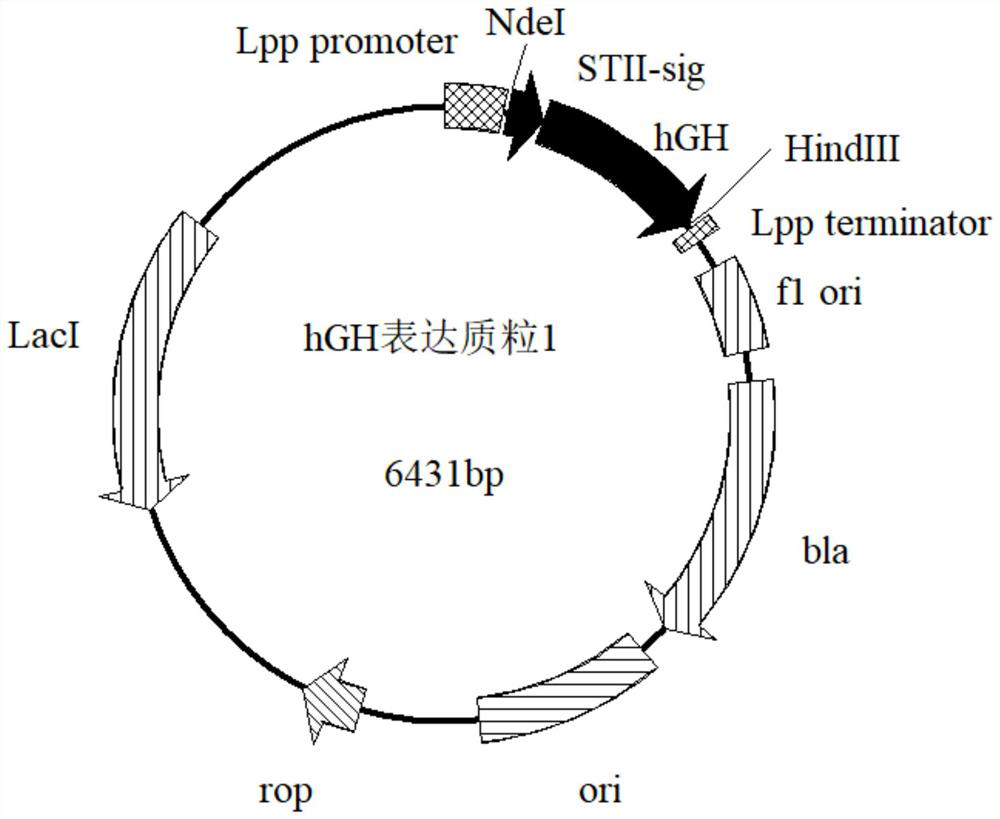
[0042] 2. Construction of expression plasmids and transformation of host bacteria
[0043] The signal peptide of the outer membrane protein of Escherichia coli is modified, and the expression of hGH is guided by the selected signal peptide of the outer membrane protein of Escherichia coli as a leader peptide, wherein the designed signal peptide is shown in SEQ ID NO:2.
[0044] Digest the synthetic ST-II-hGH gene (BGI Synthesis) with NdeI and HindIII enzymes to form sticky ends, and use 1% agarose gel electrophoresis to recover the target gene;
[0045] The PeT22b200211 expression vector was digested wit...
Embodiment 2
[0049] 1. Optimize the acquisition of genes
[0050] According to the known natural amino acid sequence of hGH, according to the codon preference of Escherichia coli and considering the elimination of hairpin structure and other secondary structures that are not conducive to expression, the coding sequence of hGH protein is optimized, as shown in SEQ ID NO: 1 DNA sequence.
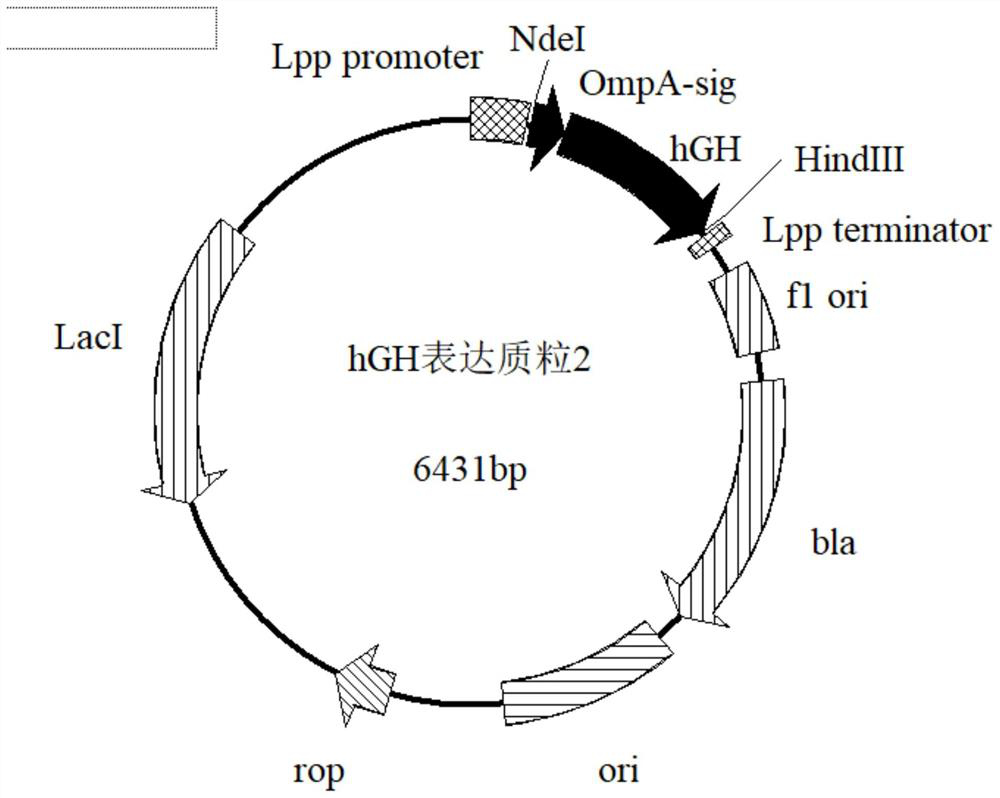
[0051] 2. Construction of expression plasmids and transformation of host bacteria
[0052] The signal peptide of the outer membrane protein of Escherichia coli is modified, and the expression of hGH is guided by the selected signal peptide of the outer membrane protein of Escherichia coli as a leader peptide, wherein the designed signal peptide is shown in SEQ ID NO:3.
[0053] Digest the synthetic OmpA-hGH gene with NdeI and HindIII enzymes to form sticky ends, and use 1% agarose gel electrophoresis to recover the target gene;
[0054] The pET22b200211 expression vector was digested with NdeI and HindII...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com