Tag peptide capable of realizing affinity binding with polystyrene and method for preparing enzyme-linked immuno solid phase antigen with same
A polystyrene, enzyme-linked immunosorbent technology, applied in the field of tag peptides, can solve problems such as the inability of enzyme-linked immunization to generate an effective immune response, the inability of directional binding of antigens, and the difficulty in achieving high sensitivity, so as to improve the sensitivity of ELISA detection and avoid Effects of conformational changes or site masking, improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
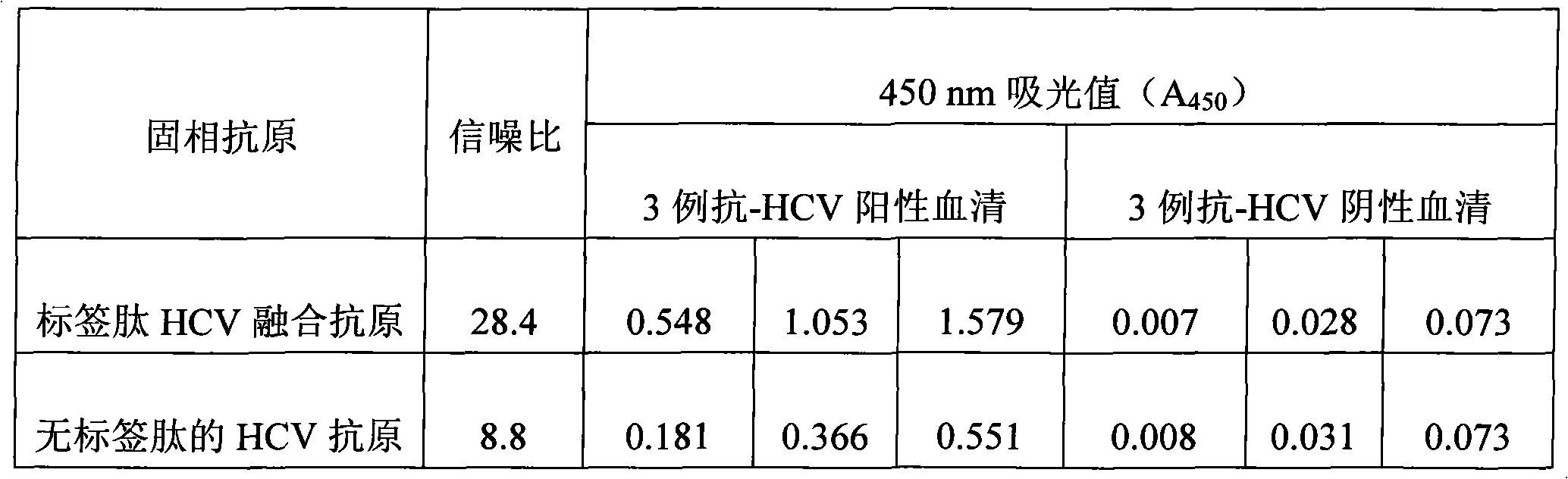
[0020] Example: Application of label peptide-guided fusion antigen site-directed immobilization in anti-HCV (hepatitis C virus, hepatitis C virus) ELISA
[0021] (1), construction of label peptide HCV fusion antigen expression plasmid
[0022] A pair of complementary single-stranded DNA (5'-TCGAG TTC AAA TTC TGG CCA TAC GAA CAT GTA ATA CGG GGG C-3' and 5'-TCGAG CCC CCG TAT TAC ATG TTC GTA TGG CCA GAA TTT GAA C-3', the underlined part is the coding sequence of the tag peptide) after incubation at 95°C for 15 minutes and annealing (cooling to 30°C within 60 minutes), the product (Xho I-Lig2) was inserted into the Xho I restriction site of the HCV antigen prokaryotic expression plasmid pET-hcv point, named pET-hcv-Lig2 after sequencing verification, which is the tag peptide HCV fusion antigen expression plasmid.
[0023] (2), expression and purification of tag peptide HCV fusion antigen
[0024] Transform the constructed tag peptide HCV fusion antigen expression plasmi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com