Applications of recombinant high-density lipoprotein in treating hypertensive disorders in pregnancy
A technology for high-density lipoprotein and gestational hypertension, which is applied in the application field of recombinant high-density lipoprotein in the treatment of gestational hypertension, can solve the problems of a large amount of plasma, high production cost, and cannot completely replace HDL therapy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of recombinant high-density lipoprotein (rhHDL)
[0023] a. Take 4mg of lecithin and 10mg of vitamin E, dissolve in 0.5ml of absolute ethanol. Inject quickly into 12.1ml of PBS after inhalation with a skin test syringe, N 2 Stir for 15 minutes;
[0024] b. Dissolve 5.4mg sodium cholate in 0.5ml PBS; dissolve 10mg rhApoA-I, 5mg rhApoE and 4mg lecithin in 0.8ml PBS. Add to the above liquid under stirring;
[0025] c. Place at room temperature for 30 minutes; move to 4°C and incubate for 12 hours, each component will polymerize to form rhHDL;
[0026] d. Add the liquid into a protein dialysis bag with a pore size of 10 kD, and fully dialyze the dialysate (PBS) at 4° C. to remove sodium cholate.
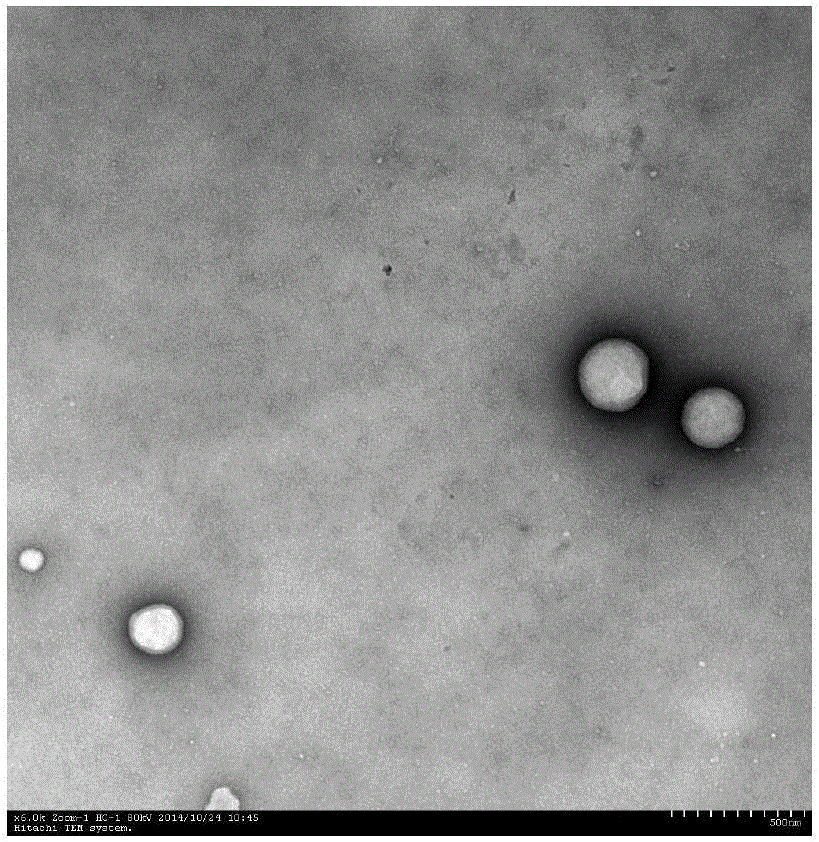
[0027] TEM observation of e.rhHDL (see attached figure 1 ).
[0028] The results of transmission electron microscopy showed that vitamin E, various apolipoprotein components, lecithin, cholesterol, etc. were successfully polymerized into spherical r...
Embodiment 2
[0029] Example 2: Effect of rhHDL on placental oxidative stress damage and pregnancy outcome in preeclampsia mice
[0030] 1) Replication of preeclampsia mouse model
[0031] Healthy adult sexually mature ApoE - / - 60, 20 C57BL / 6J, male and female, 8-10 weeks old. Raise them in a barrier system with a room temperature of 20-25°C and a relative humidity of 40%-70%, with 12 hours of day and night lighting, and no restriction on food and water. Male and female mice were caged overnight at 1:00 at 10:00 the night before, and the vaginal suppository was observed before 7:00 the next morning. If the vaginal suppository is observed on the first day of pregnancy, it will be kept in isolation. C57BL / 6J pregnant mice served as the normal control group and were given standard feed; ApoE - / - Pregnant mice were randomly divided into three groups, model control group, rhHDL low-dose group and rhHDL high-dose group, and fed high-fat diet.
[0032] From the 11th day of pregnancy, ApoE - / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com