Serum/plasma protein molecular marker related to auxiliary diagnosis of intrahepatic cholestasis in gestation period and application thereof
A molecular marker, cholestasis technology, applied in the fields of genetic engineering and reproductive medicine, to achieve the effect of rapid and accurate diagnosis, convenient and easy diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The collection of embodiment 1 sample and the arrangement of sample data
[0052] The inventor collected a large number of peripheral blood samples of ICP patients and healthy control pregnant women from October 2016 to September 2017 from the Wuxi Maternal and Child Health Hospital affiliated to Nanjing Medical University (the samples used for research were collected during the same period, sampled, sub-packaged, and stored) The conditions are uniform), and by sorting out the sample data, the inventor selected 100 samples that meet the following criteria as experimental samples for DIA protein quantitative detection and subsequent series of enzyme-linked immunosorbent assays and automatic biochemical analyzer verification:
[0053] 1. The above research objects are second trimester Pregnant women who were confirmed to have ICP during ICP screening (refer to the guidelines for diagnosis and treatment of ICP patients (first edition)) were defined as cases.
[0054] 2. ...
Embodiment 2
[0056] Example 2 DIA protein quantitative detection of protein molecular markers in serum / plasma
[0057] The above-mentioned 10 eligible ICP cases and 10 healthy controls were quantitatively detected by DIA protein to obtain relevant results. The specific steps are:
[0058] 1. Sample Preparation
[0059] 1.1 Add an appropriate amount of SDT lysate to the blood sample, transfer it to Lysing Matrix A tube, and use MP homogenizer for homogenization and crushing (24×2, 6.0M / S, 60s, twice).
[0060] 1.2 After ultrasonication, take a boiling water bath for 10 minutes. Centrifuge at 14000 g for 15 min, take the supernatant and filter it with a 0.22 μm centrifuge tube, and collect the filtrate.
[0061] 1.3 BCA method was used for protein quantification. Aliquot the samples and store at -80°C.
[0062] 2. SDS-PAGE electrophoresis
[0063] 20 μg of protein from each sample was added to 6X loading buffer, boiled in water for 5 minutes, subjected to 12% SDS-PAGE electrophoresis (...
Embodiment 3
[0091] Verification experiment of protein molecules in embodiment 3 serum / plasma
[0092] 1. ELISA
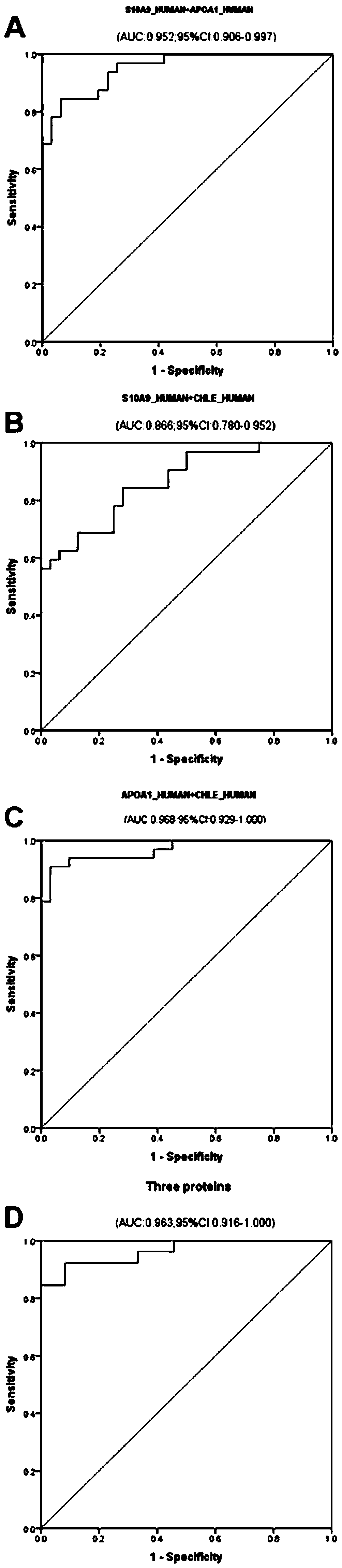
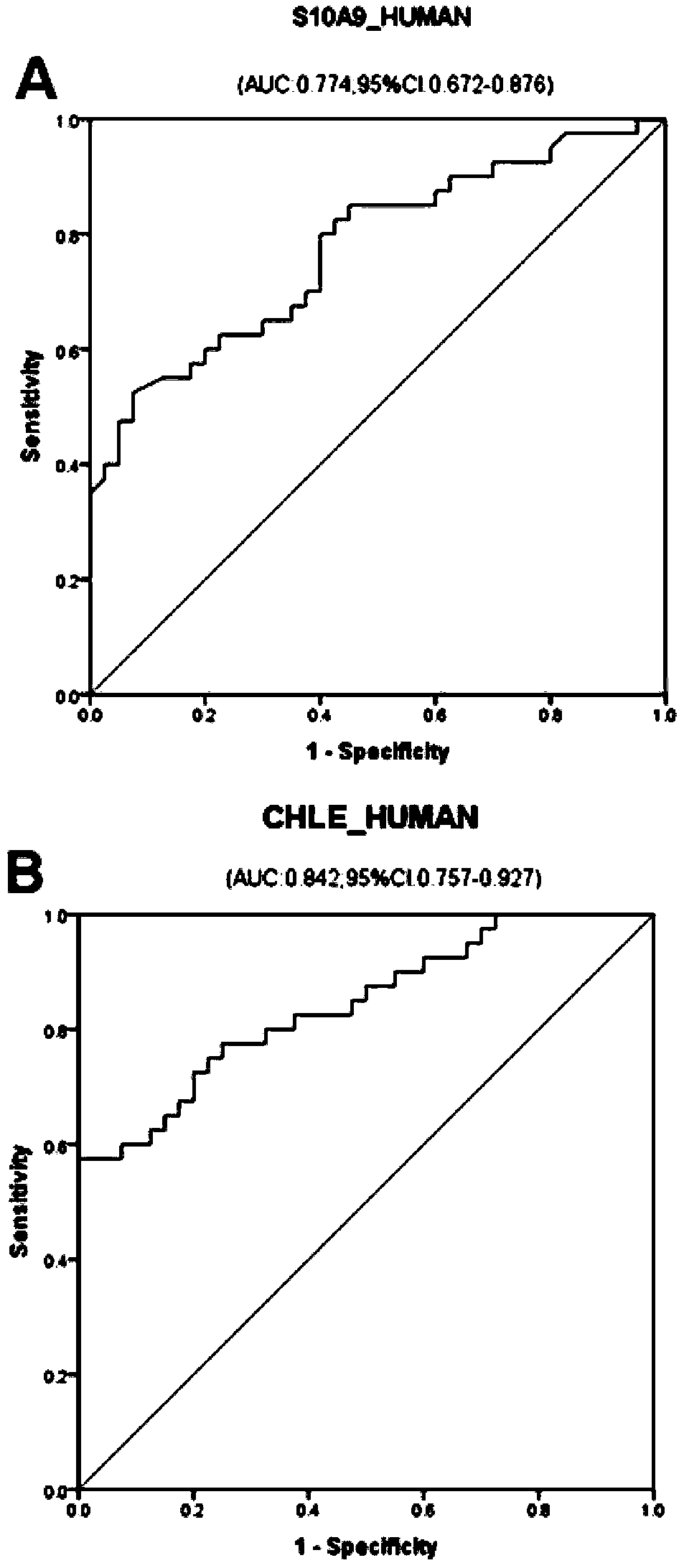
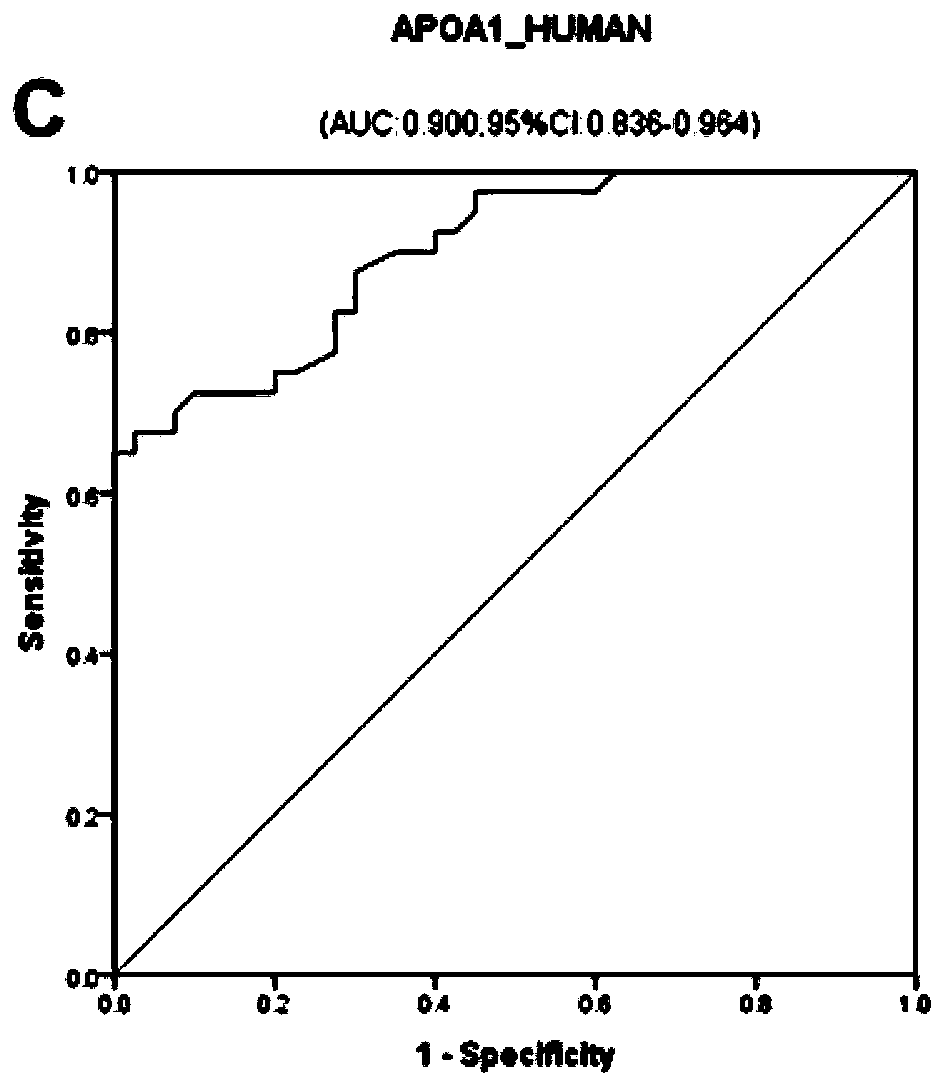
[0093] Select 50 cases of ICP patients and 50 cases of healthy controls who meet the conditions of Example 1, detect the expression levels of protein molecular markers S10A9_HUMAN and ITIH3_HUMAN in the serum / plasma samples of the two groups by enzyme-linked immunosorbent assay, analyze and compare the differences, and draw ROC curve. The specific method is:
[0094] 1.1 Dilution of the standard product: This kit provides a standard product at original times, and the user can dilute it in a small test tube according to the following table.
[0095]
[0096] 1.2 Adding samples: Set up blank wells (blank control wells do not add samples and enzyme-labeled reagents, and the rest of the steps are the same), standard wells, and sample wells to be tested. Accurately add 50 μl of the standard substance on the enzyme-labeled plate, add 40 μl of the sample diluent to the well of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com