Method of expressing human apolipoprotein ApoA I inside Pichia yeast cell
A technology of apolipoprotein and intracellular expression, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, Construction and Identification of Recombinant Expression Plasmids
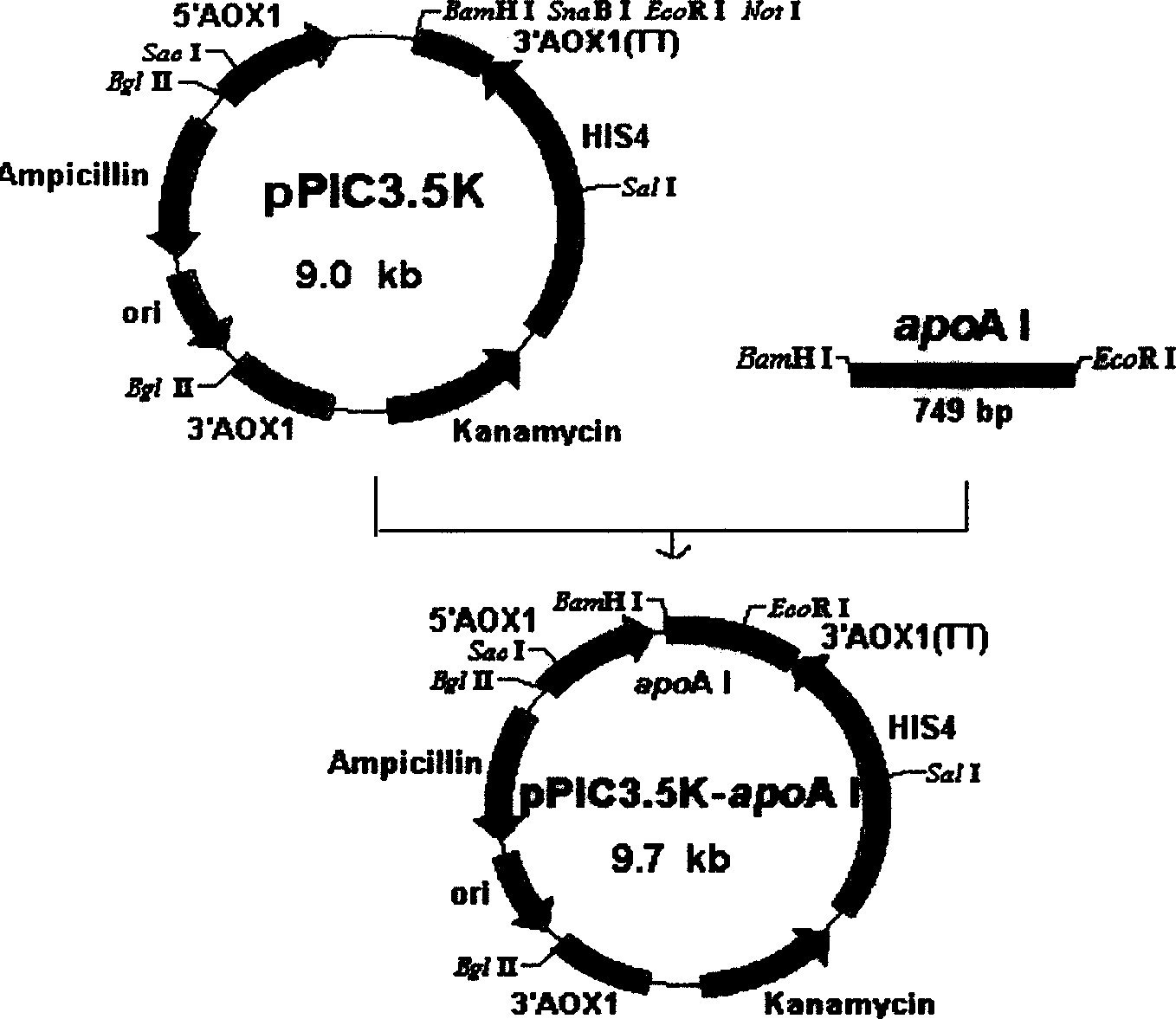
[0024] Using the plasmid pPIC9k-apoA I with the artificially synthesized gene apoA I as a template, primers were designed for PCR amplification. Forward primer: 5'-GGGGATCCAAACGATGGATGAACCACCTCAGTCTCCATGG-3', BamH I site was introduced at the 5' end, reverse primer: 5'-GCAAATGGCATTCTGACATCC-3'. The PCR reaction conditions were set according to conventional settings. The PCR product was double-digested with EcoR I and BamH I, and the intracellular expression plasmid pPIC3.5k was also double-digested with EcoR I and BamH I, followed by ligation and transformation. The recombinant expression plasmid pPIC3.5k-apoA I was extracted from the transformant, identified by enzyme digestion and DNA sequence determination, which proved that the construction of the recombinant expression plasmid was completely correct.
Embodiment 2
[0025] Example 2, Electrotransformation of recombinant expression plasmid Pichia GS115
[0026] Inoculate Pichia yeast GS115(his4) in YPD medium, shake culture at 28-30°C until OD600=1.3-1.5, centrifuge, collect the bacteria, wash once with pre-cooled sterile water and 1mol / L sorbitol, Finally, 200 μl of 1mol / L sorbitol was used to suspend to obtain GS115 competent cells. Take 80 μl of GS115 competent cells and mix them with 5 μg of recombinant expression plasmid digested with Bgl II, transform by electric shock under the conditions of 1300V, 25μF, 200Ω, suspend with 1ml of 1mol / L sorbitol, spread the RDB plate, and incubate at 28-30℃ After 3-4 days, more than 1400 transformants were obtained as a result.
Embodiment 3、G418
[0027] Example 3, Screening of G418 highly resistant yeast transformants
[0028] More than 1,400 colonies of yeast transformants grown on RDB plates were planted on YPD plates with G418 concentrations of 1.5, 3.0, and 4.0 mg / ml, cultured at 28-30°C, and G418-resistant strains were screened step by step. Results 27 strains were obtained on the plate containing 4mg / ml G418.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com