Anti-human apoa1 monoclonal antibody and its preparation method and application
A monoclonal antibody, cardiovascular and cerebrovascular disease technology, applied in biochemical equipment and methods, chemical instruments and methods, microbial-based methods, etc., can solve problems such as the inability to detect changes in HDL subtypes, and achieve risk prediction and Effects of clinical monitoring, improvement of sensitivity, and increase of detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
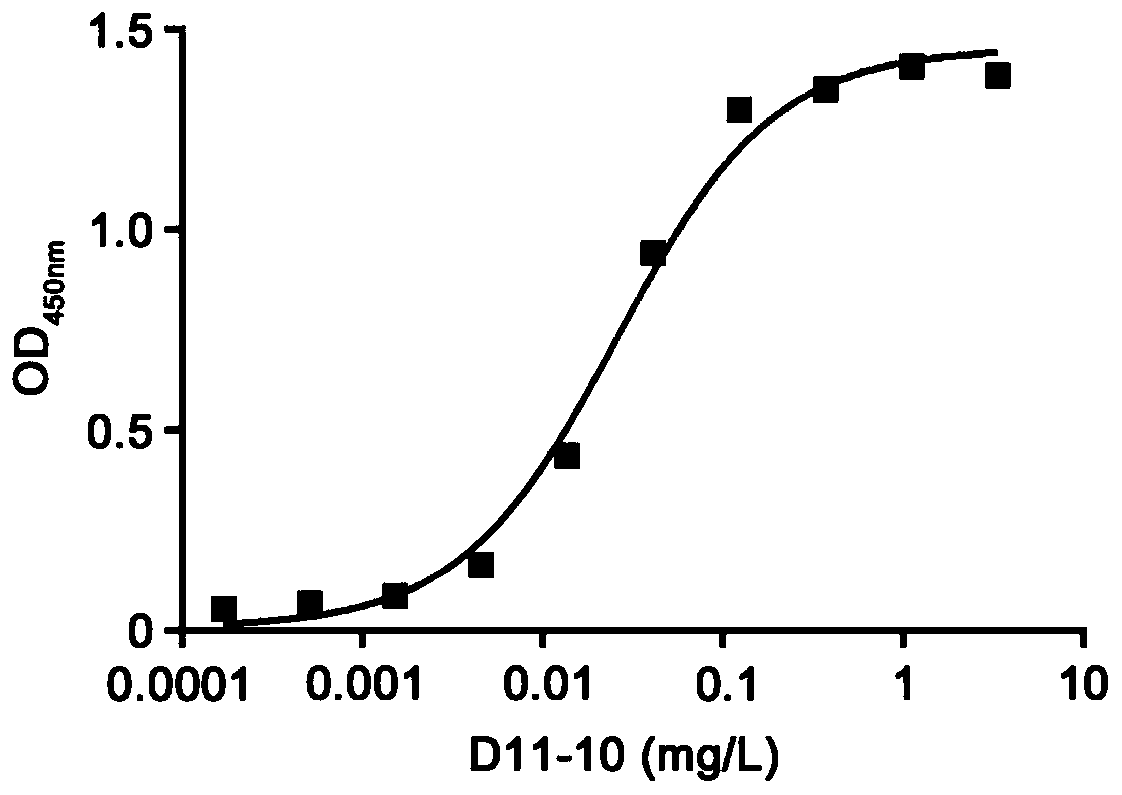
[0029] Example 1: Preparation and purification of ApoA1 monoclonal antibody D11-10
[0030] 1. Materials: fusion protein 6×His-ApoA1, 8-week-old female BALB / c mice
[0031] 2. Methods and Results
[0032] 2.1 Antigen preparation
[0033] 2.1.1 Construction of human ApoA1 recombinant protein expression plasmid
[0034] The gene sequence (Gene ID: 335) of ApoA1 was obtained by querying in GenBank, and polymerase chain reaction (PCR) primers were designed according to the sequence. The DNA fragment of human ApoA1 gene was amplified using human cDNA as a template. The PCR product was detected by 1.0% agarose gel electrophoresis and the corresponding fragment was recovered with a gel recovery kit. The size of the ApoA1 gene fragment amplified by PCR was 875bp. The PCR product was ligated into the expression vector pCold TM II (the 6×His tag contained in the vector is used for purification), transformed into TaKaRaDH5α host bacteria, picked a single clone for plasmid extraction an...
Embodiment 2
[0051] Example 2: Determination of the variable region sequence of ApoA1 monoclonal antibody D11-10
[0052] 1. Materials: Trizol (Invitrogen), primers were synthesized by Sangon Bioengineering Company, reverse transcription and PCR reagents were purchased from TaKaRa Company.
[0053] 2. Methods and results:
[0054] 2.1 Total RNA extraction and cDNA first-strand synthesis
[0055] The hybridoma cells in the logarithmic growth phase were collected, and total RNA was extracted according to the operating procedure of the Trizol manual. The qualitative and quantitative identification of total RNA was performed using a spectrophotometer and agarose gel electrophoresis.
[0056] According to TaKaRa PrimeScript TM II 1st Strand cDNA Synthesis Kit Instructions Synthesize cDNA.
[0057] 2.2 Gene fragment amplification and sequencing of ApoA1 monoclonal antibody D11-10 heavy chain variable region (VH) and light chain variable region (VL)
[0058] According to TaKaRa company's Taq...
Embodiment 3
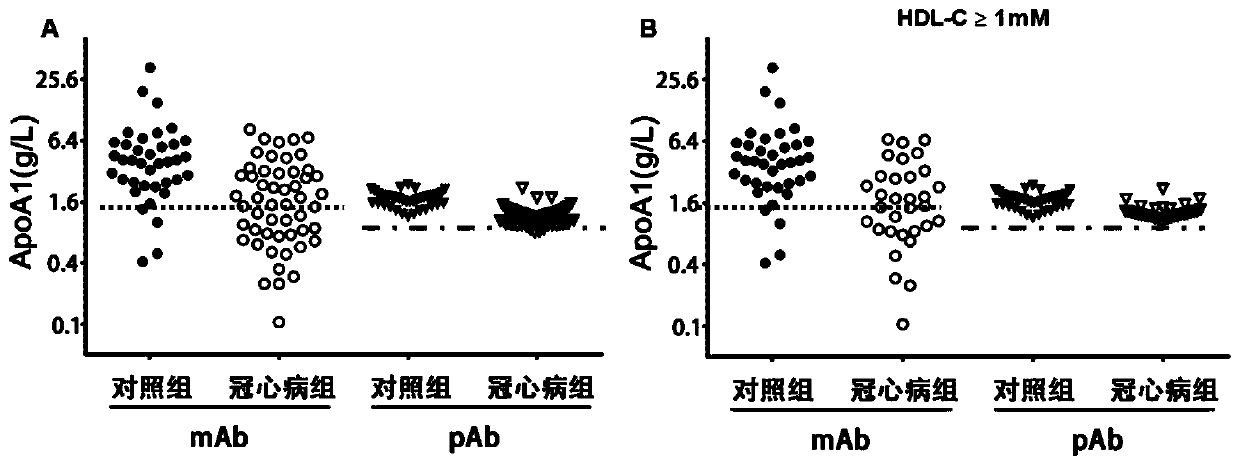
[0062] Example 3: Clinical application of the detection method established based on ApoA1 monoclonal antibody D11-10 in the risk prediction and diagnosis of cardiovascular and cerebrovascular diseases
[0063] 1. Materials:
[0064] ApoA1 monoclonal antibody D11-10; serum samples from healthy people (no history of coronary heart disease) and patients with coronary heart disease (CHD).
[0065] 2. Methods and results:
[0066] 2.1 Preparation and assignment of mixed serum calibrator
[0067] Serum samples from 20 healthy individuals were used, and an equal volume of serum was taken from each case into centrifuge tubes (100 μL each), placed at 4°C for thorough mixing, then aliquoted in 200 μL / tube, and frozen in a -80°C refrigerator. One was taken out the next day, thawed at 4°C, and after equilibrating to room temperature, the ApoA1 immunoturbidimetric kit from Desai Company was used to assign values to the mixed serum samples. The concentration of ApoA1 in the mixed serum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com