Human apolipoprotein AI genetic engineering preparation method and expression vector and engineering bacteria thereof
A technology of genetically engineered bacteria and apolipoproteins, applied in the field of DNA recombination for the production of proteins or polypeptides, can solve the problems of low expression amount, high expression cost, complicated purification steps, etc., to maintain biological activity, overcome instability, and simplify The effect of purification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
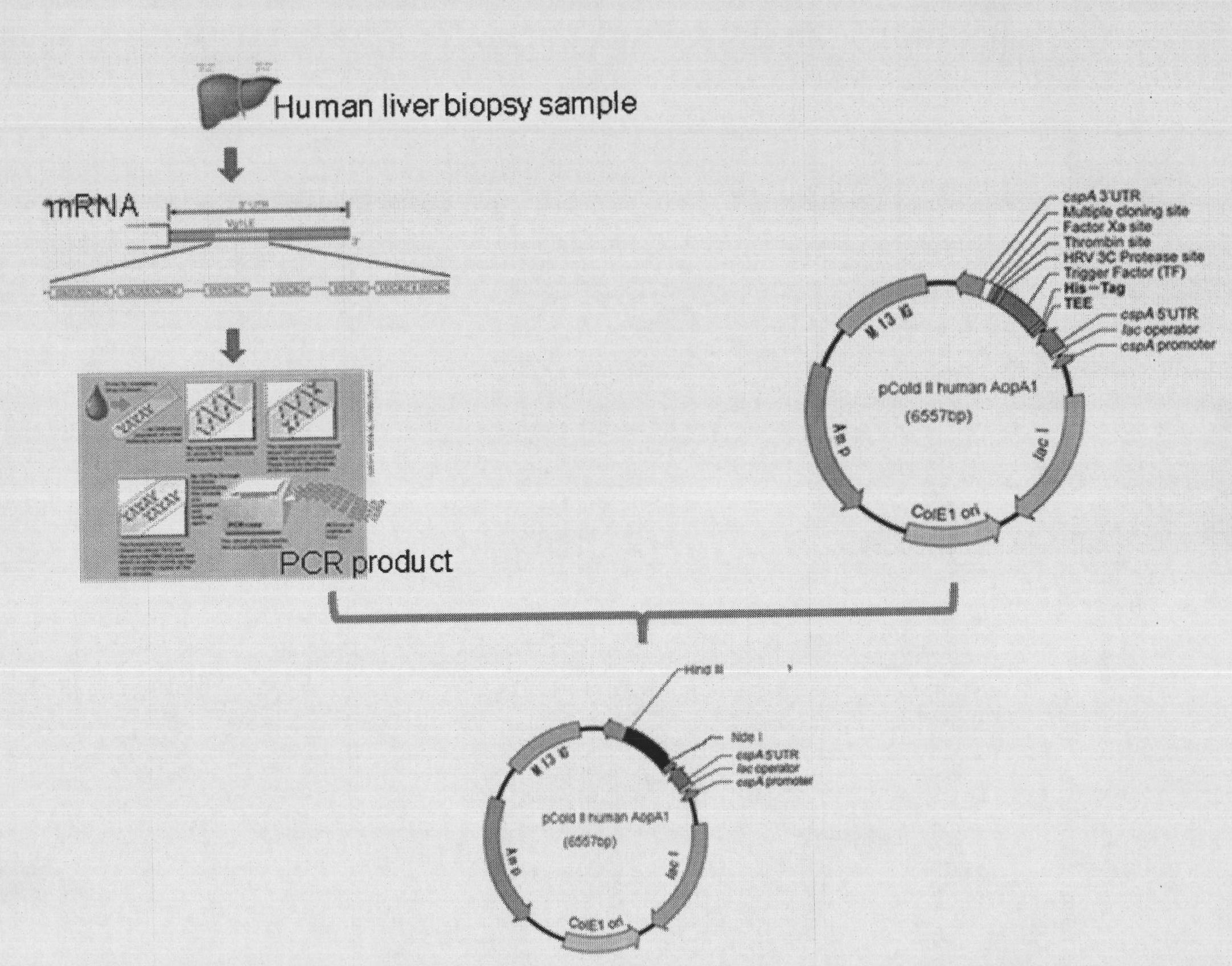
[0043] Example 1 PCR amplification of the coding gene of human apolipoprotein AI and construction of recombinant expression plasmid pCold-HuApoA1:
[0044] 1) PCR amplification of the target gene
[0045] ① Extraction of total RNA: Take fresh liver tissue from the patient, grind it with a homogenizer, and extract total RNA with an RNA extraction kit
[0046] ② RT-PCR: Using total RNA as a template, use a reverse transcription kit to obtain the eDNA of human apolipoprotein AI.
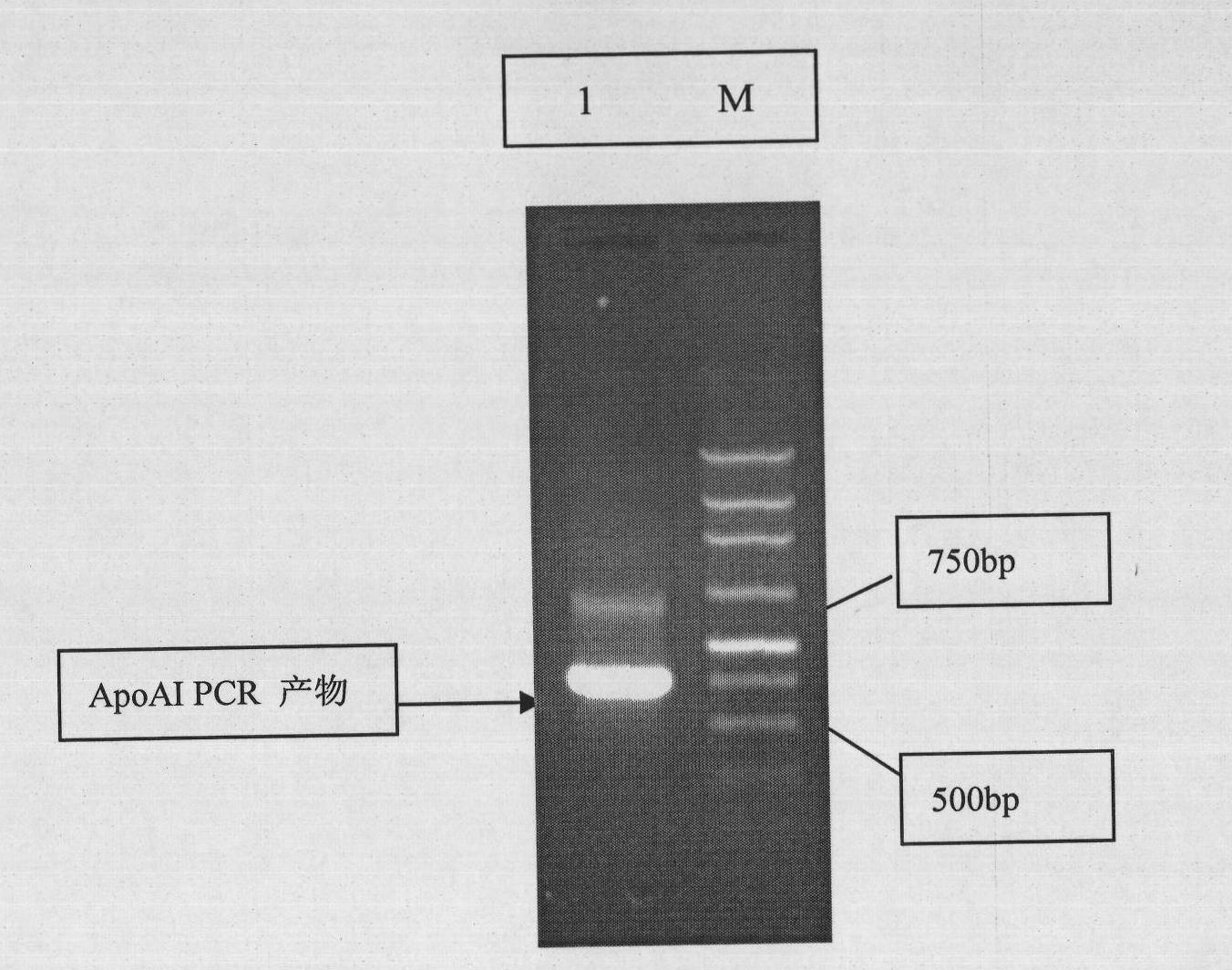
[0047] ③PCR amplification of the target gene: Design a pair of primers according to the human apolipoprotein AI sequence published in the gene bank. The primers are:
[0048] Upstream primer: 5'AAT CATATG GATGAACCCCCCCCAGAGCCCCT(NdeI)
[0049] Downstream primer: 5'AT AAGCTT CTGGGTGTTGAGCTTCTTAGTGTA(HindIII)
[0050] Referring to the reported human apolipoprotein AI sequence, the primers were designed from the 73rd nucleotide of cDNA, thereby artificially removing the signal peptide and precursor pept...
Embodiment 2
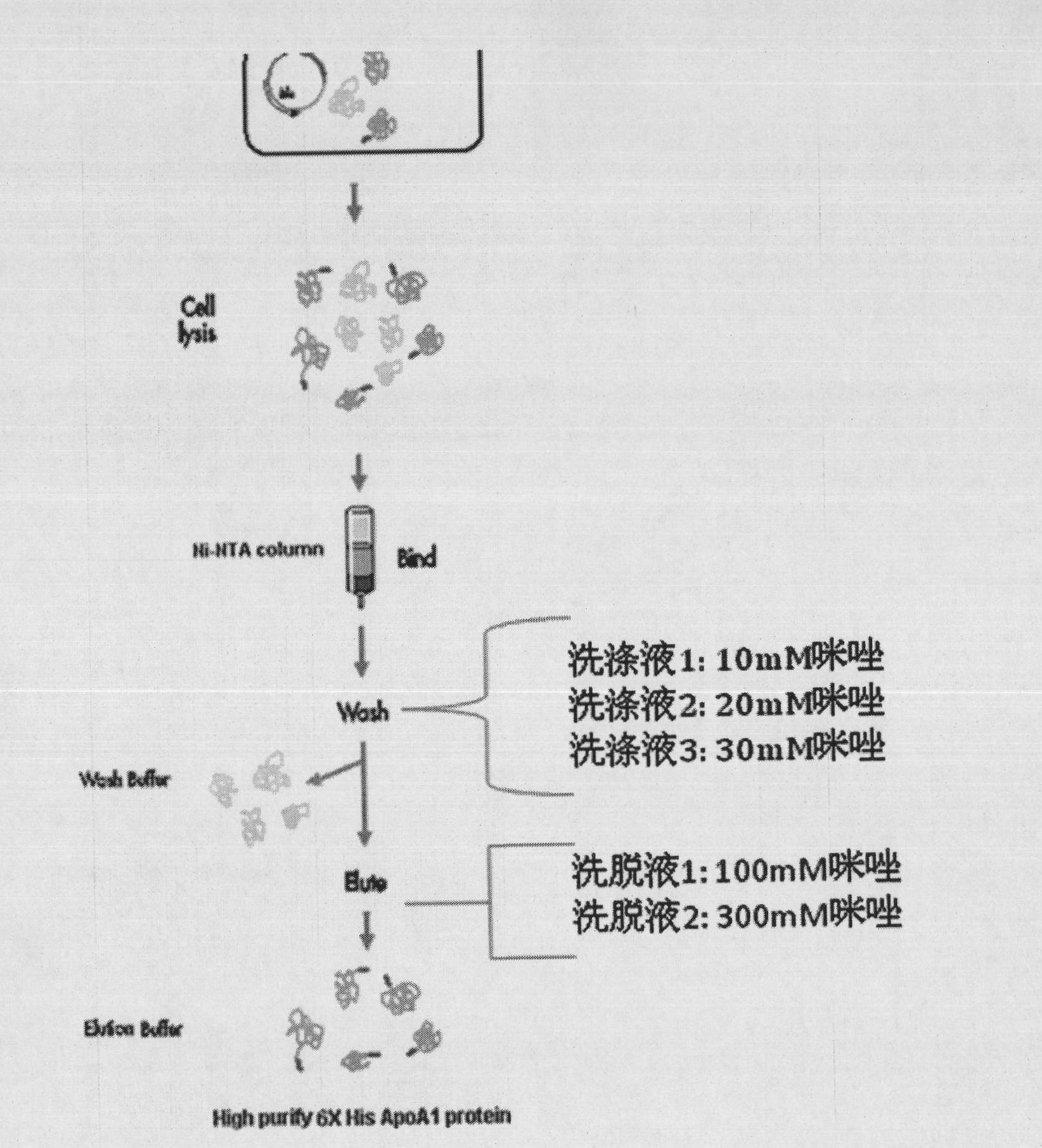
[0067] Large-scale expression and purification method of embodiment 2 gene recombinant bacteria
[0068] 1) Expression of recombinant human ApoA1-polyhistidine fusion protein in Escherichia coli host
[0069] ①Transform pCold-ApoA1 into Rosetta-gami Escherichia coli on LB plates with ampicillin resistance (100ng / ml), culture overnight at 37°C
[0070] ②Pick a single colony in 50ml LB culture containing 100μg / ml ampicillin at 37°C, 250 rpm, and culture overnight
[0071] ③ 2% inoculated into 1L 2YT medium (including 100ug / ml ampicillin), 37℃to A600, 0.D.0.5
[0072] ④Reduce the cultivation temperature to 10-20℃, and let the bacteria solution continue to cultivate for 30 minutes
[0073] ⑤ Add IPTG to a final concentration of 1mM 10°C, 300rpm induction culture for 15hours
[0074] ⑥Centrifuge at 4000rpm for 20 minutes at high speed, collect the precipitate and store it at -80°C
[0075] 2) Reagent for purifying recombinant human apolipoprotein AI-polyhistidine fusion protein...
Embodiment 3
[0096] Proof of Example 3 Recombinant Protein (15% SDS-acrylamide gel electrophoresis and Western blot method)
[0097] detailed steps:
[0098] 1) 15% SDS-acrylamide gel, through Tris-glycine buffer, 90V voltage, 3 hours electrophoresis, detect the finally obtained recombinant protein. The detection result shows that the specific band is consistent with the molecular weight of human apolipoprotein AI.
[0099] Figure 5A It is the result of electrophoresis of the total bacterial lysate of different expression host bacteria before affinity column purification. As shown by the electrophoresis results, the host bacteria BL21(DE3)PlysS, Roesetta-gami, and KS1000 transformed by the plasmid vector pCold containing the human apolipoprotein AI gene sequence were respectively induced by IPTG, compared with the samples before IPTG induction , an obvious difference band of induced protein can be seen at about 30KD, which is consistent with the expected molecular weight of human recom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com