Method for large-scale purification of recombinant human apolipoprotein Apoa-I
An apolipoprotein and large-scale technology, applied in the field of genetic engineering recombinant protein purification, can solve the problems of large volume of fermentation broth, unfavorable large-scale purification, high energy consumption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
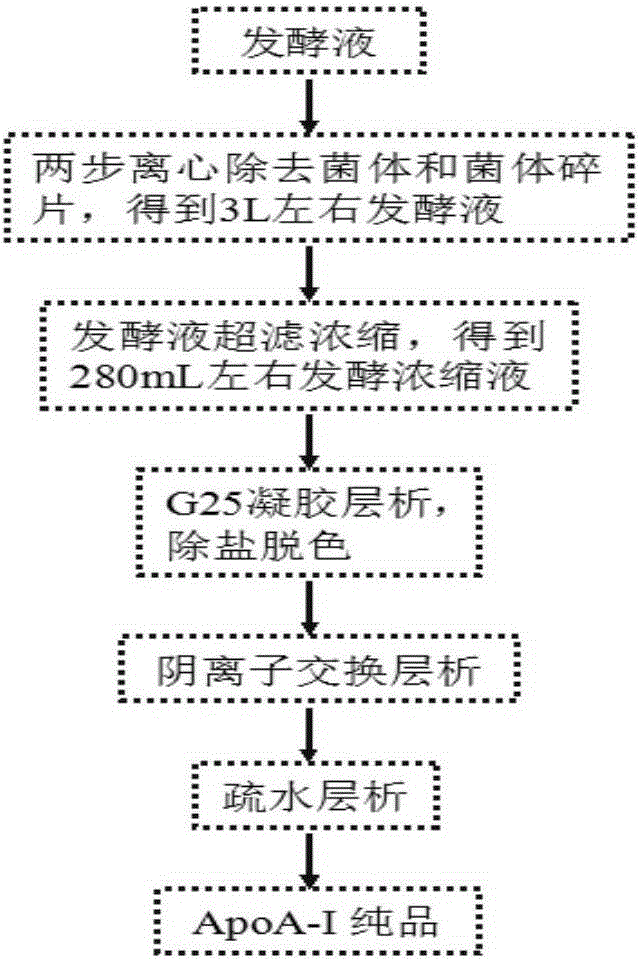
[0028] 1) Concentrate and decolorize the fermentation supernatant by ultrafiltration
[0029] Centrifuge the fermentation liquid of Pichia pastoris engineered bacteria at 4000rpm for 10min, and collect the supernatant; use ultrafiltration membrane with a molecular weight cut-off of 10K to concentrate the supernatant by ultrafiltration, and concentrate it to about 1 / 10 of the original volume; Bestdex G-25 Pack the gel filler into a 50 / 30 chromatography column, equilibrate 2 column volumes with 20mM phosphate buffer with different pH (6.5 or 7.4) (A pump of AKATAExplore protein purification instrument), flow rate is 90cm / h, and equilibrate to UV baseline Stablize;
[0030] Use the B pump to inhale 30% of the fermentation concentrate of Bestdex G-25 gel filler volume, that is, 90mL of the concentrate. After adding the sample, rinse with 20mM phosphate buffer with different pH (6.5 or 7.4), and collect the salt before the peak. UV absorption peak;
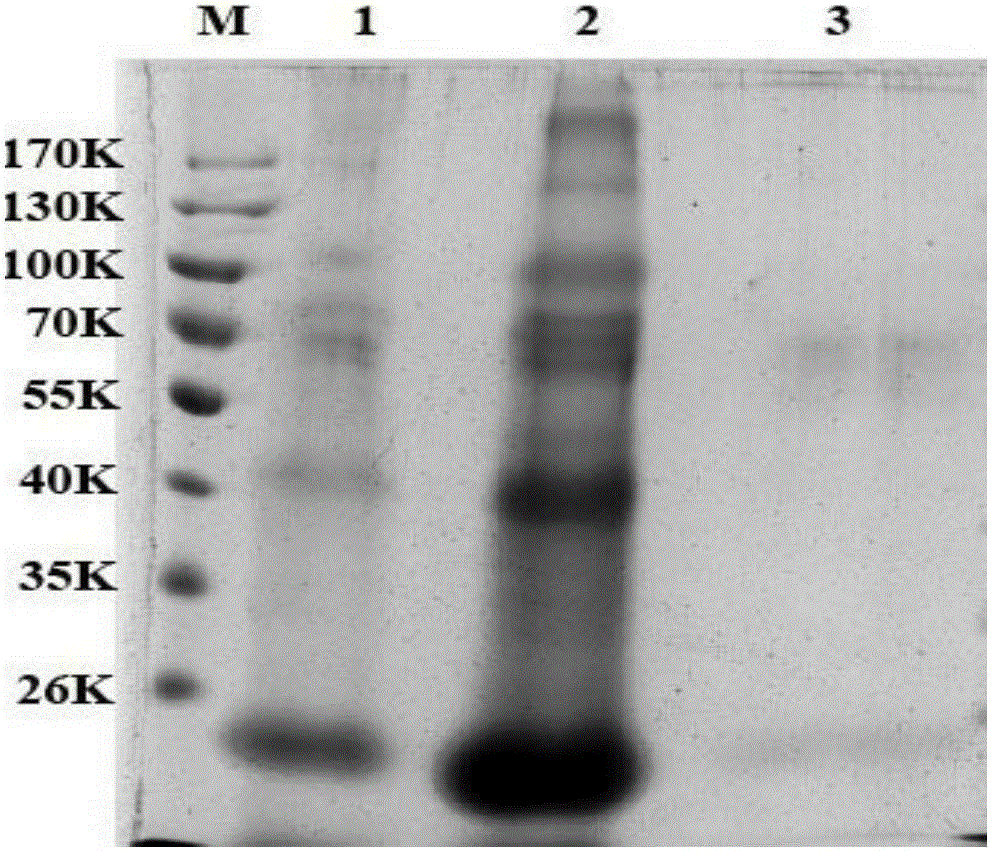
[0031] 2) Preliminary purific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com