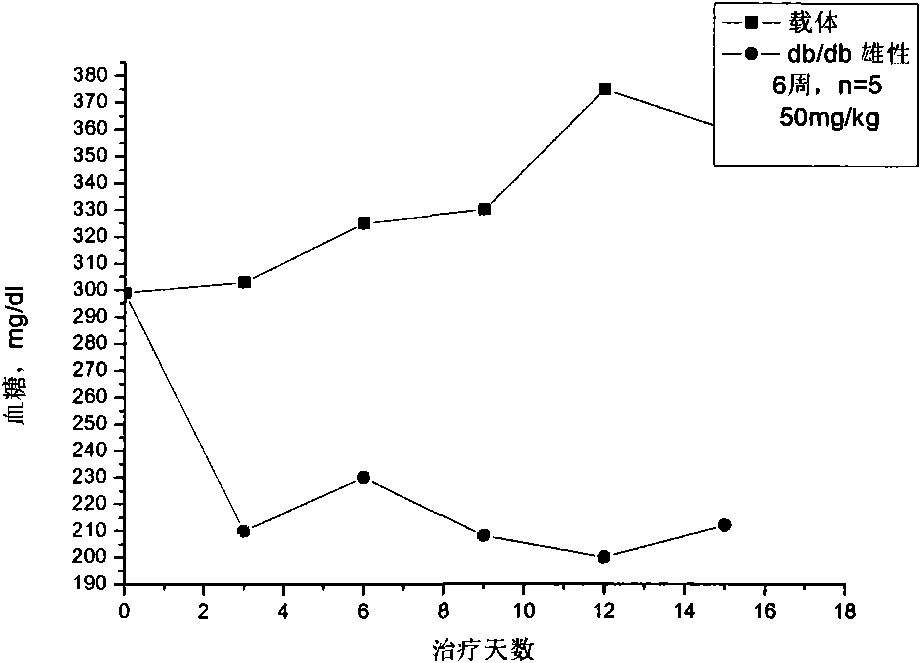
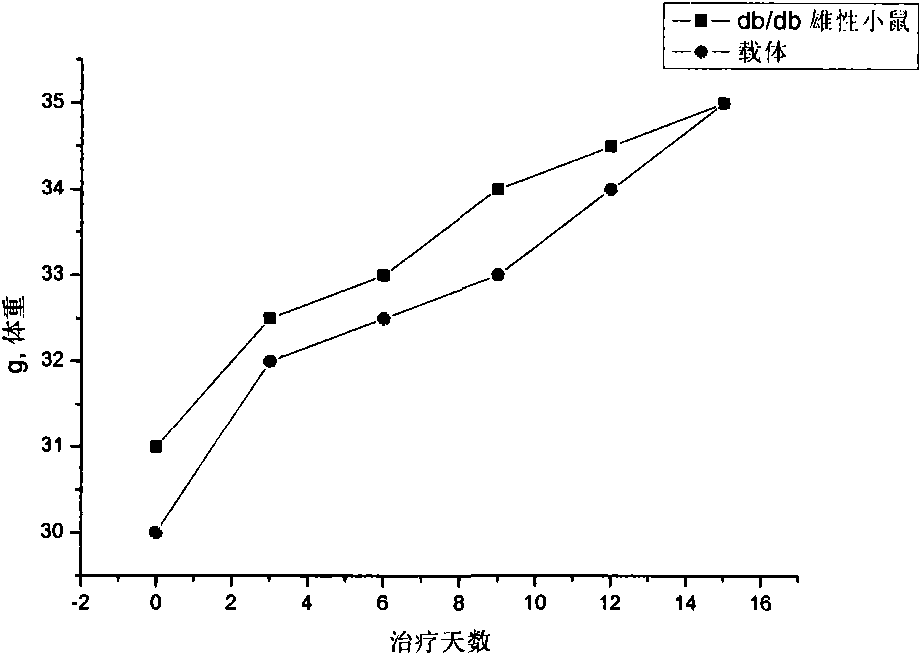
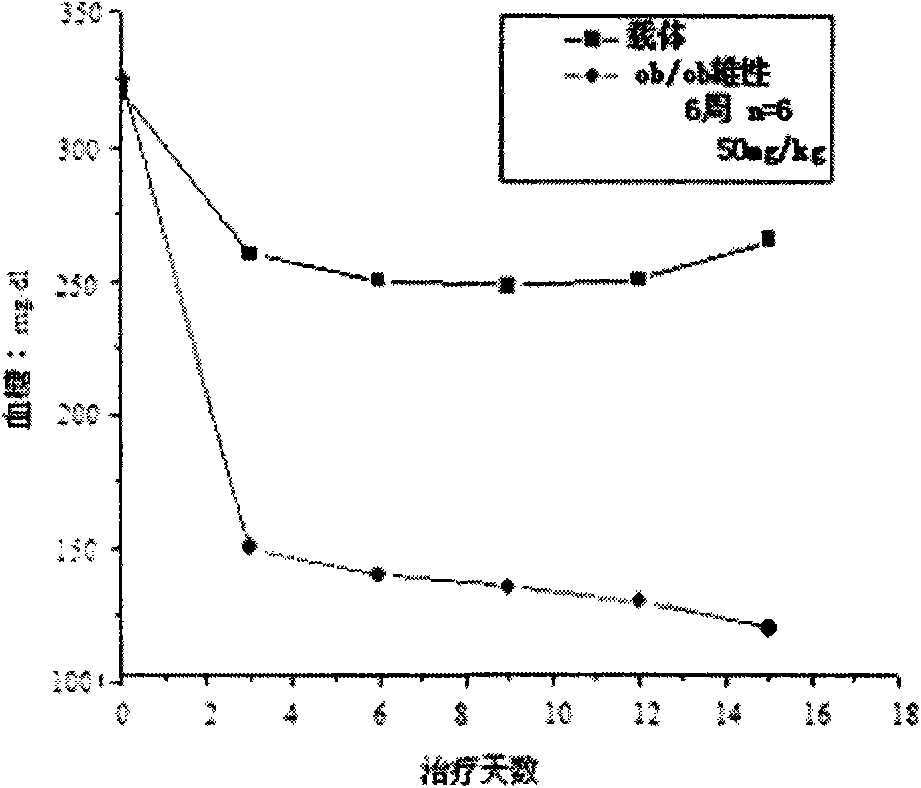
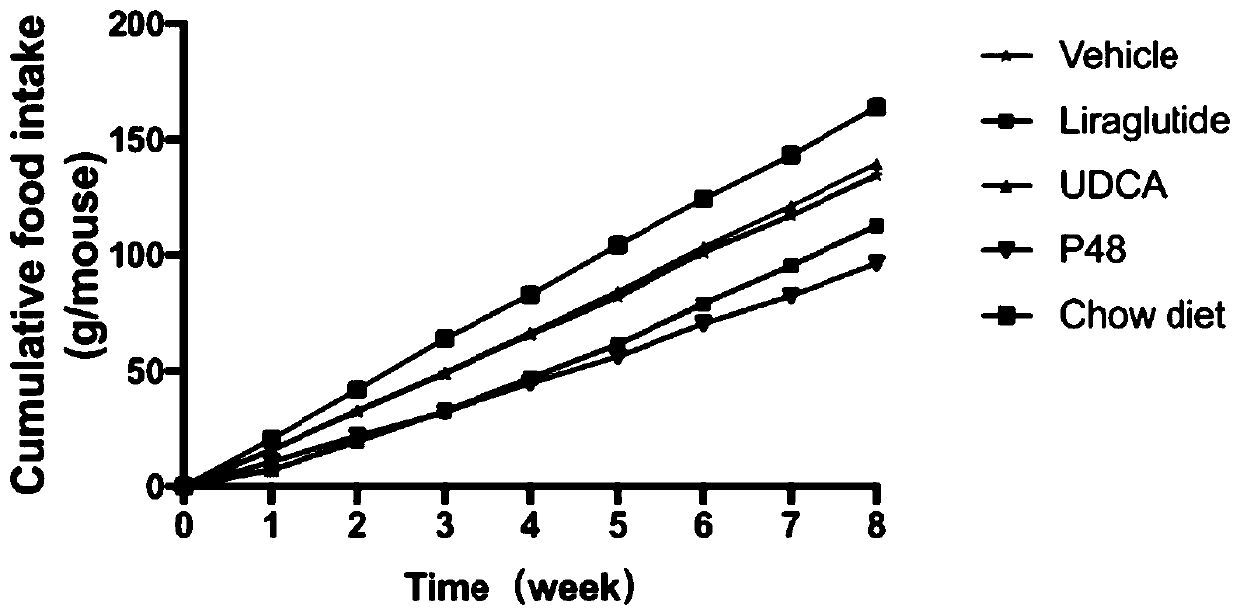
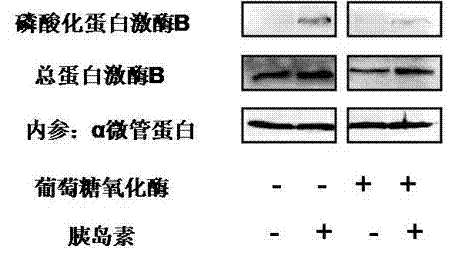
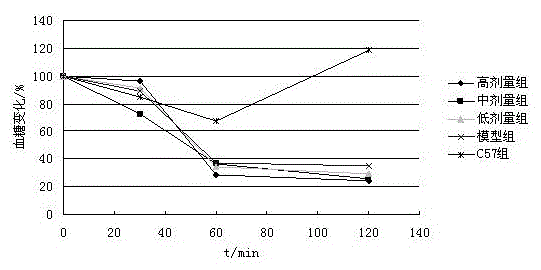
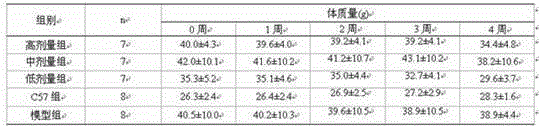
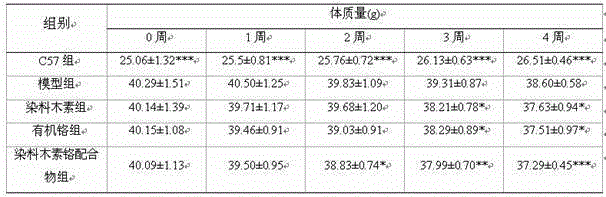
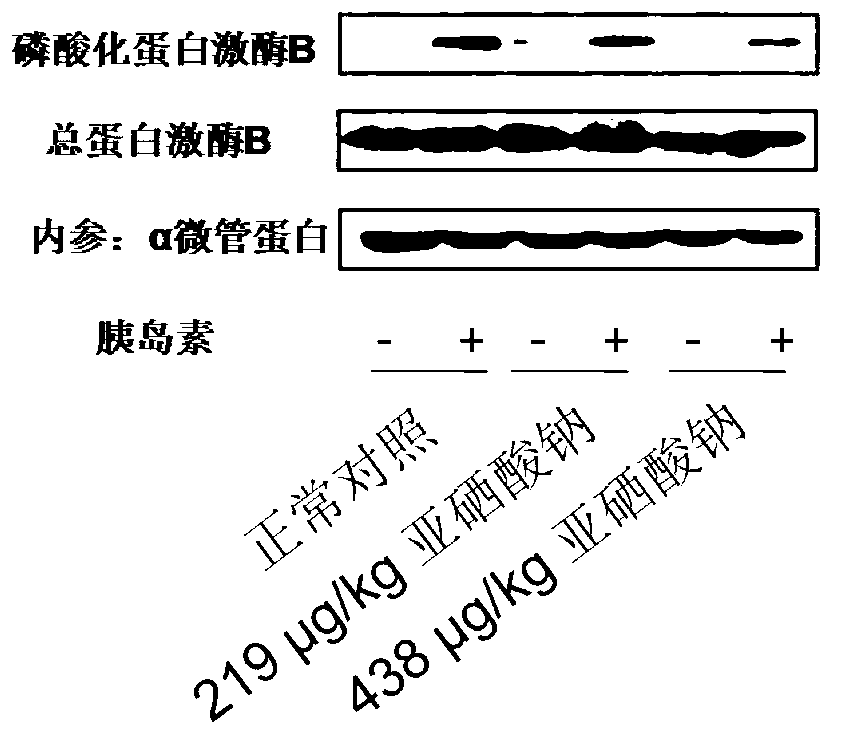
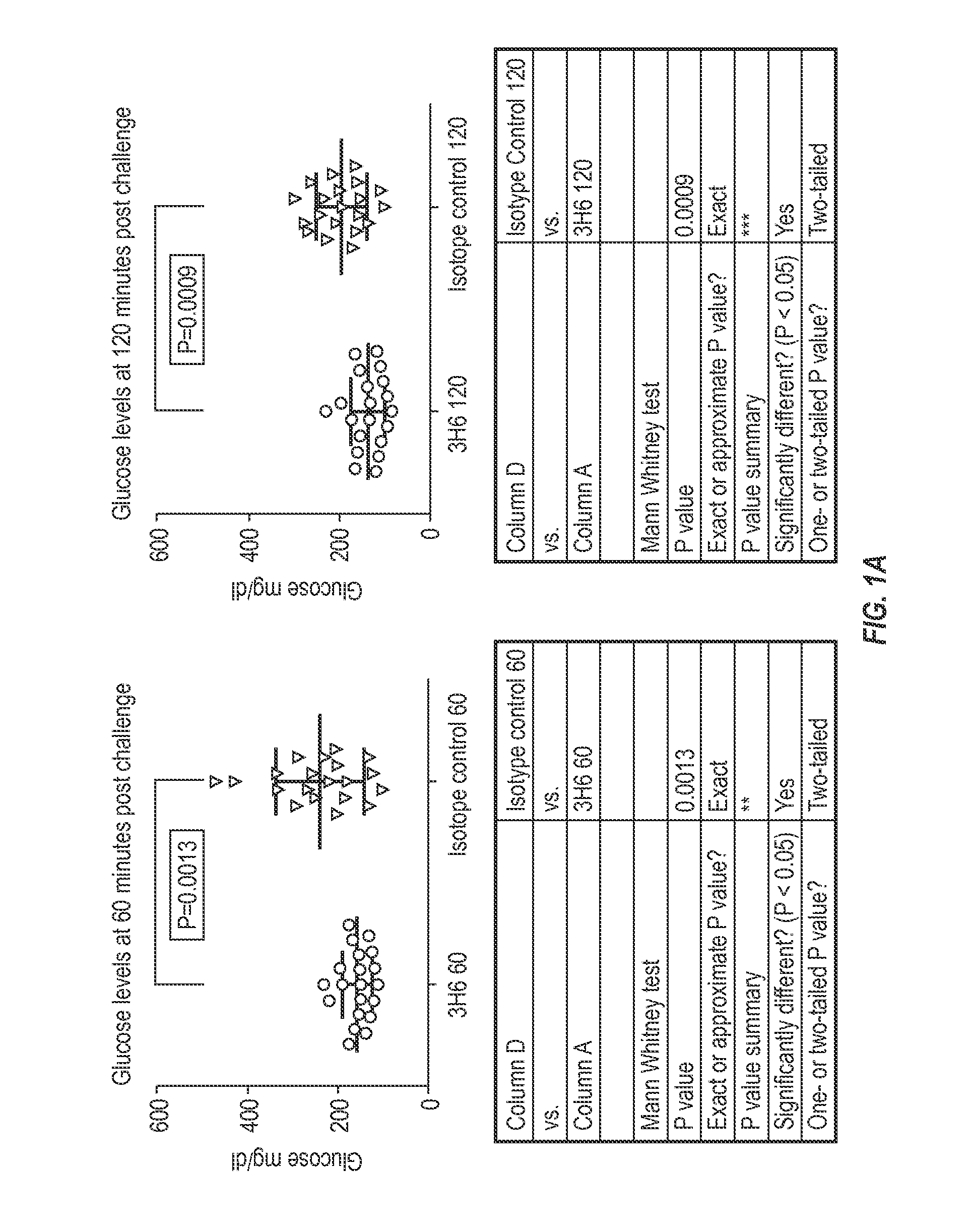
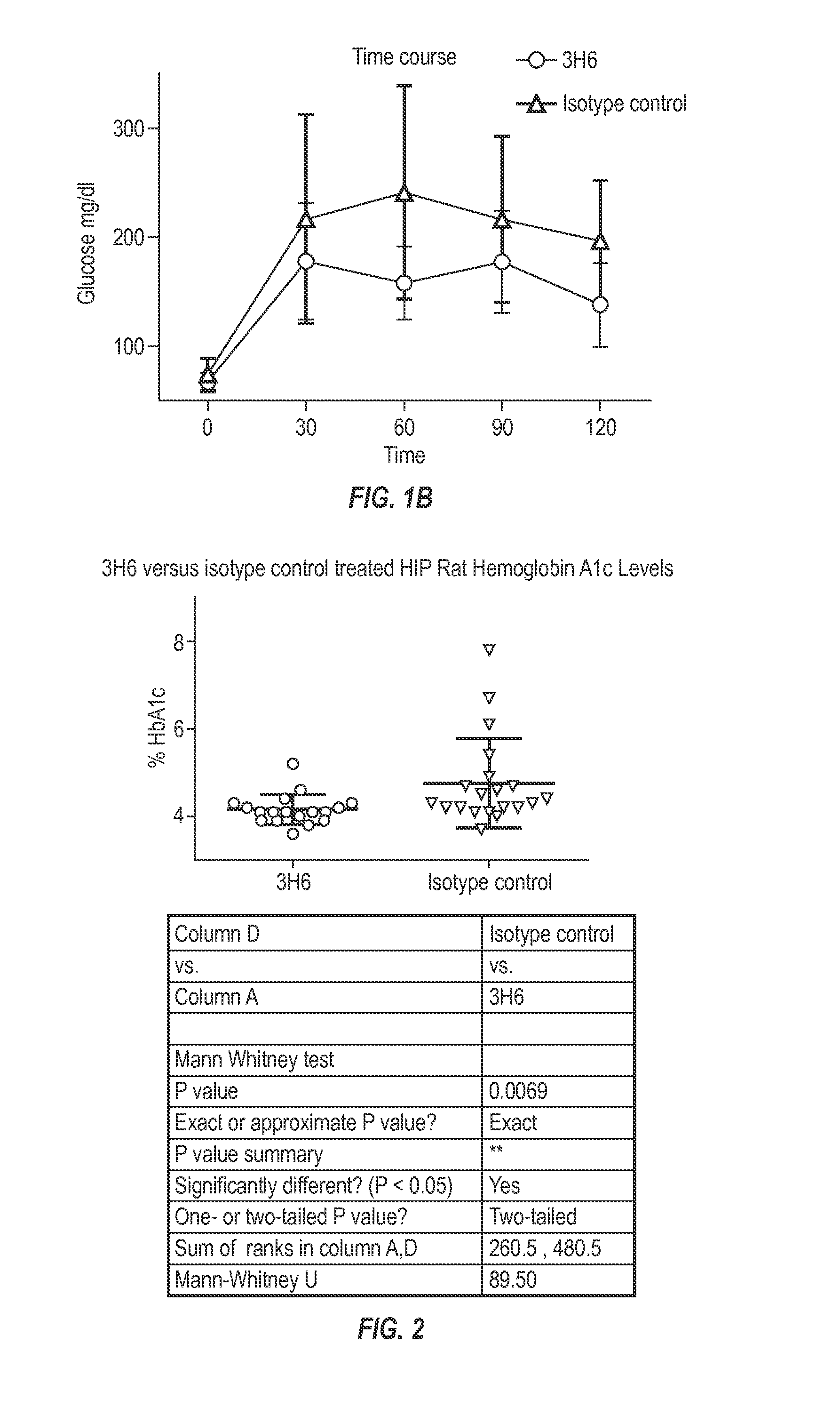
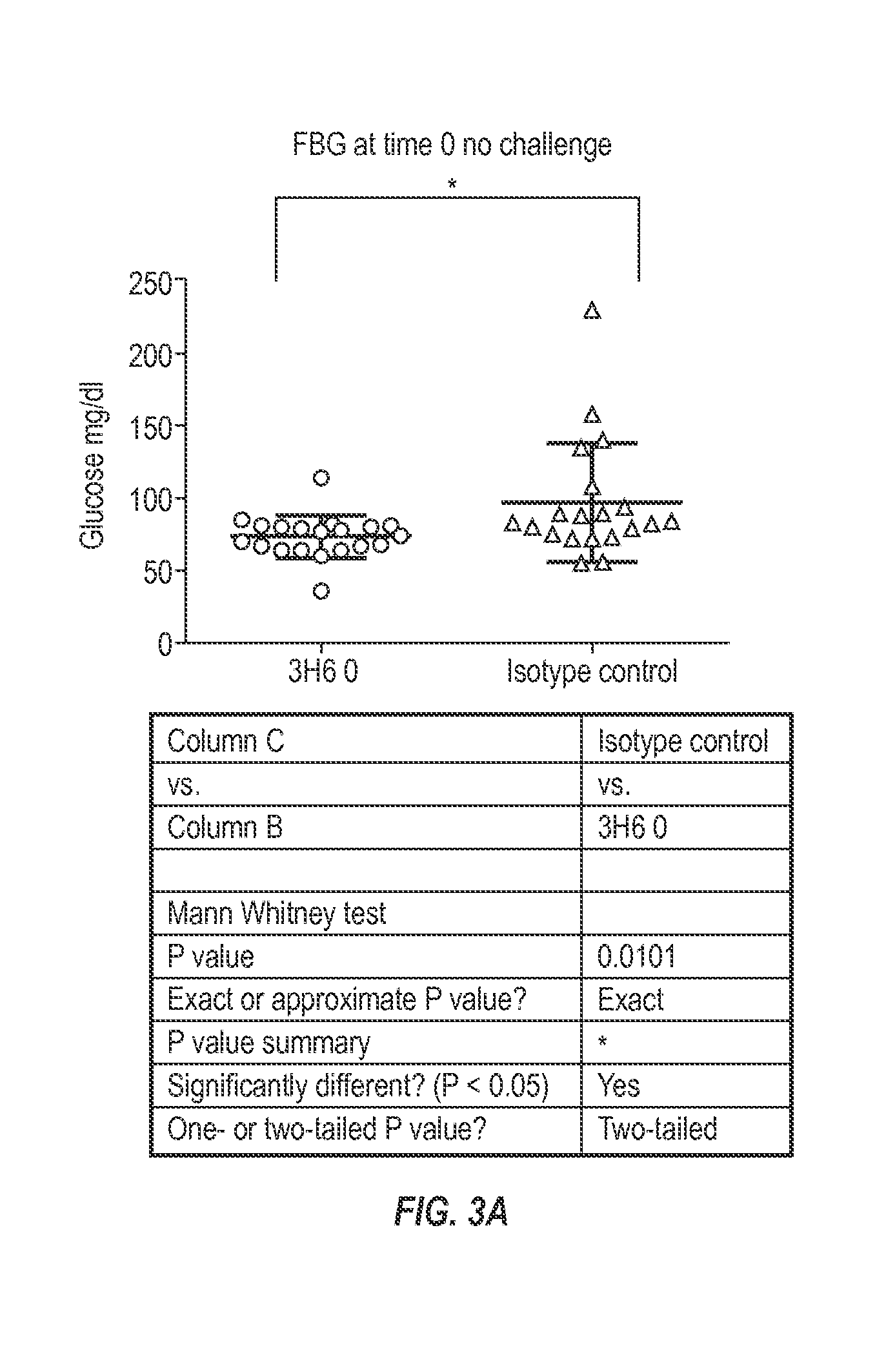
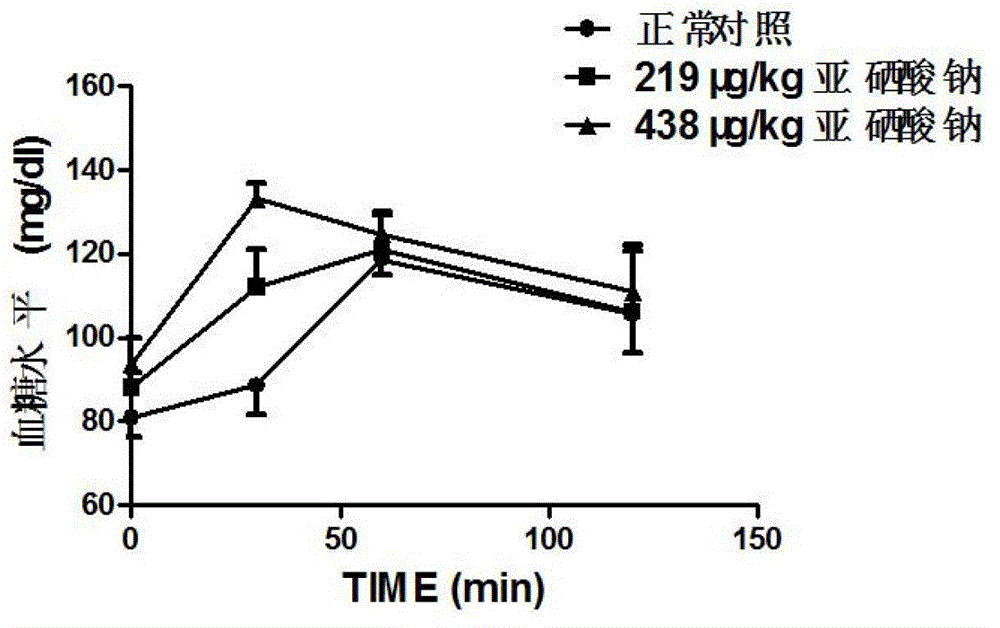
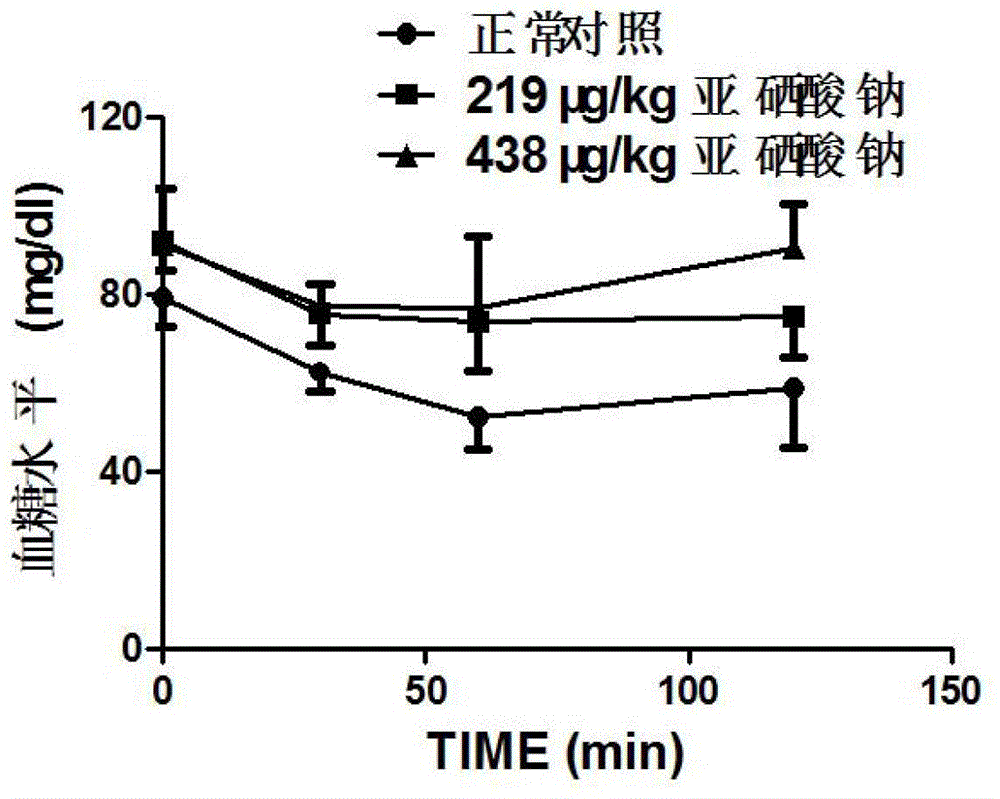
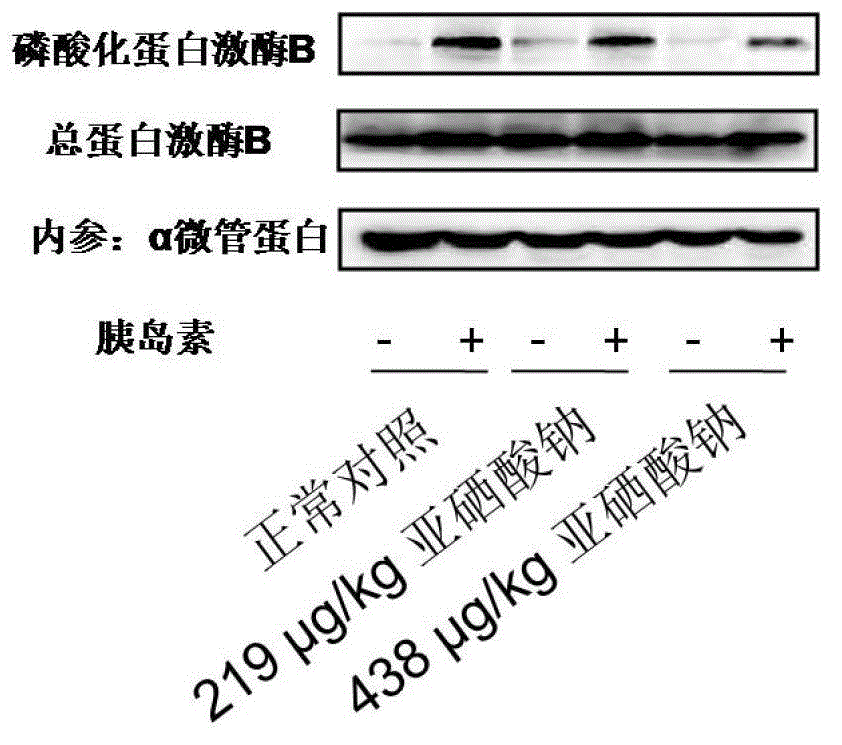
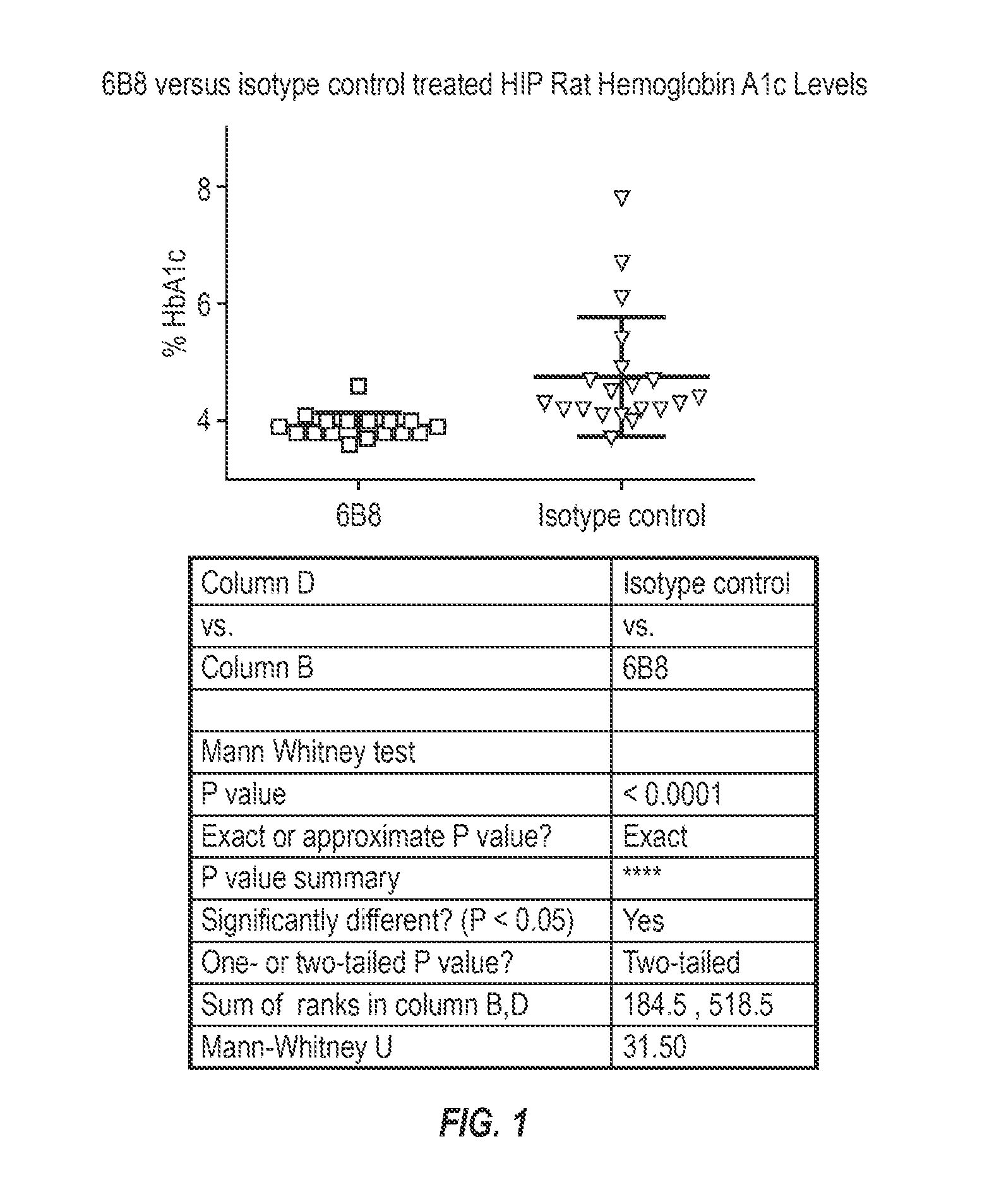
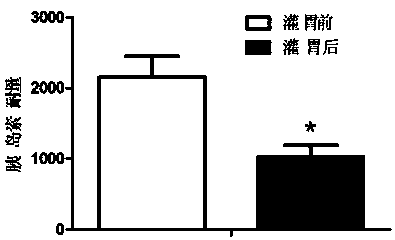
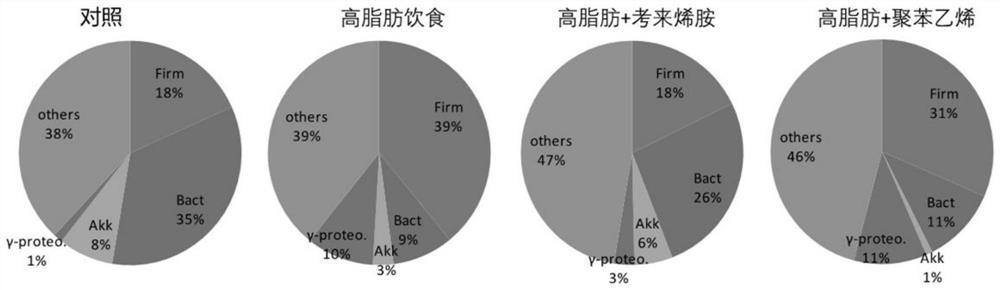
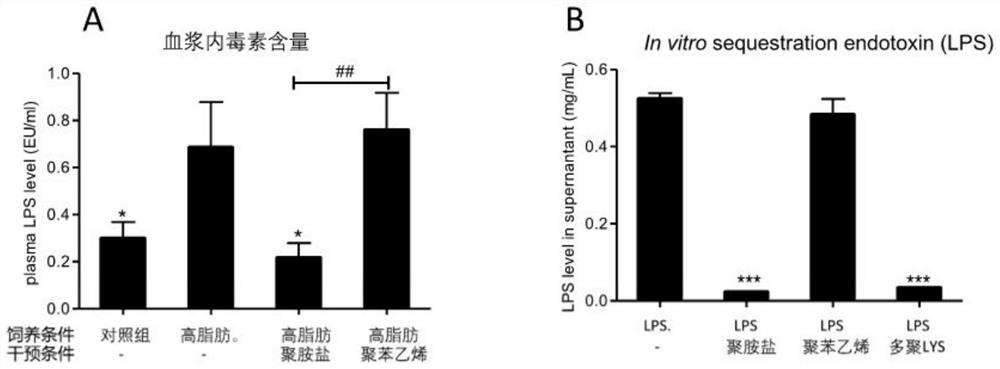
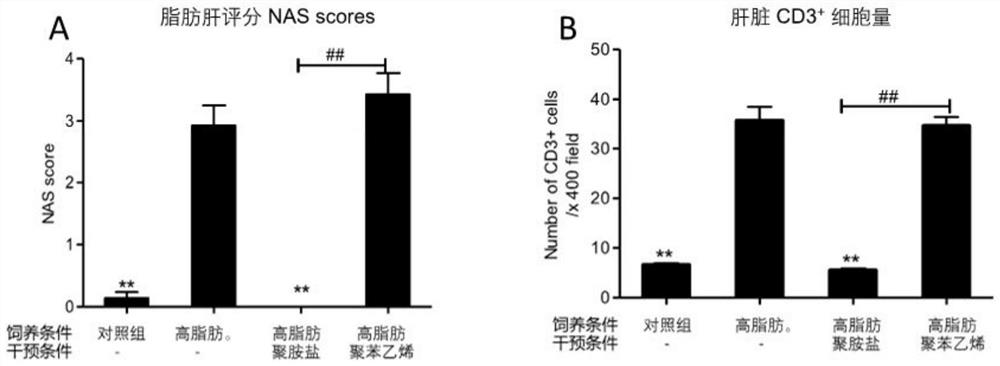
Patents
Literature
53 results about "Insulin tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
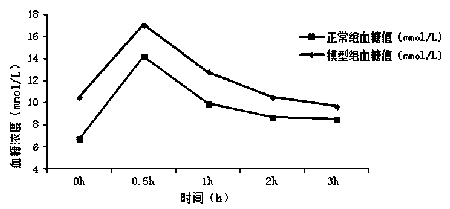
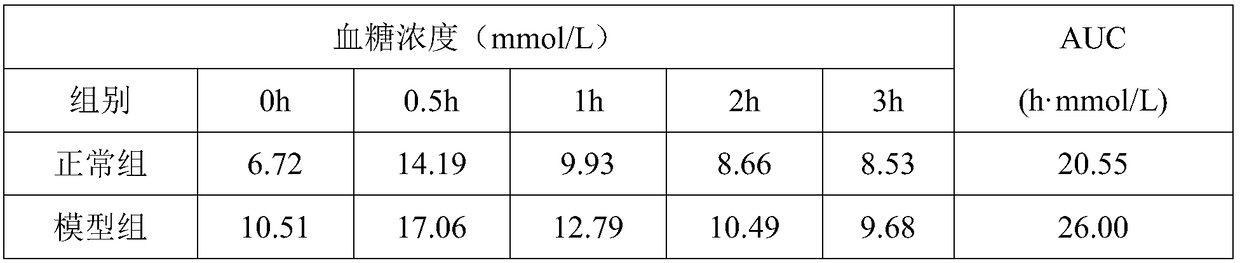
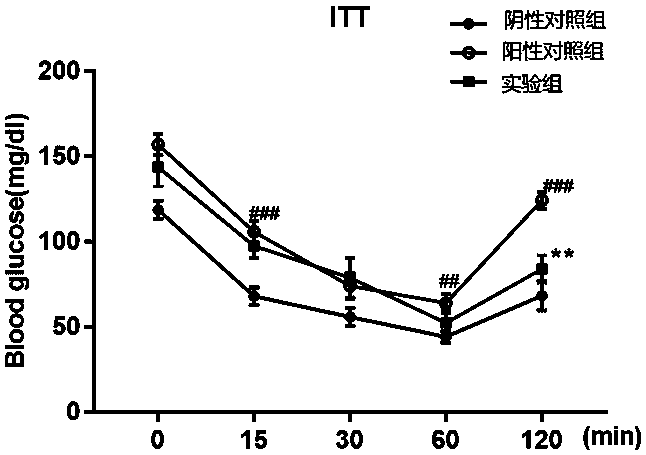
An insulin tolerance test (ITT) is a medical diagnostic procedure during which insulin is injected into a patient's vein, after which blood glucose is measured at regular intervals.
Composition For Body Fat Reduction
InactiveUS20090047304A1Reduce body fatPrevent body fat from increasingBiocideSenses disorderAdditive ingredientAstaxanthin
An increase in body fat is one of main causes of life-style-related diseases such as hypertension, insulin tolerance and hyperlipemia. A composition for body fat reduction is provided which inhibits body fat from decreasing or decreasing which and which is effective in the treatment and prevention of adult diseases a cause of which is body fat. Also, provided are a medicine and a food each containing the composition.The composition for body fat reduction comprises (A) astaxanthin and (B) at least one of active ingredient, an adsorbent and a solubilizer, a composition comprises (A) Haetmatococcusalga extract containing astaxanthin and (B) at least one of active ingredient, an adsorbent and a solubilizer, and a medicine and food, each containing the composition are provided.
Owner:FUJI CHEM IND CO LTD
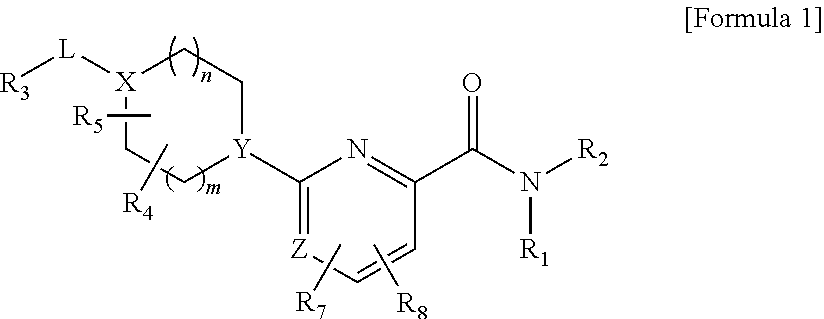
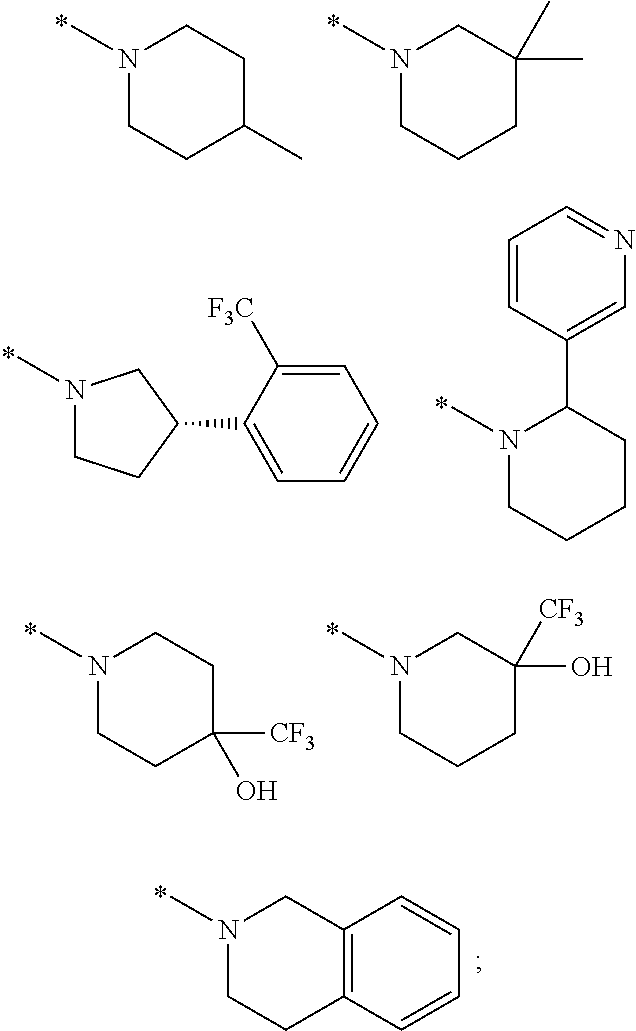
Picolinamide and pyrimidine-4-carboxamide compounds, process for preparing and phamaceutical composition comprising the same
ActiveUS20130210811A1Selective inhibitory activityImpaired glucose toleranceAntibacterial agentsBiocideDyslipidemiaGlucocorticoid
Provided are picolinamide and pyrimidine-4-carboxamide compounds, a method for preparing the same, a pharmaceutical composition containing the same, and a medical use using the compound as an agent for preventing, regulating, and treating diseases related to regulation of glucocorticoids by using selective inhibitory activity of the compound for an 11β-HSD1 enzyme. The picolinamide and pyrimidine-4-carboxamide compounds of the present invention are selective inhibitors of human-derived 11β-HSD1 enzymes, and are useful in an agent for preventing, regulating, and treating diseases related to glucocorticoid regulation in which human-derived 11β-HSD1 enzymes are involved, for example, metabolic syndromes such as, type 1 and type 2 diabetes, diabetes later complications, latent autoimmune diabetes adult (LADA), insulin tolerance syndromes, obesity, impaired glucose tolerance (IGT), impaired fasting glucose (IFG), damaged glucose tolerance, dyslipidemia, atherosclerosis, hypertension, etc.
Owner:SK CHEM CO LTD
Compositions and methods for inhibiting the enzymatic activity of PTP-1B
The present invention provides mice that have had their PTP-1B genes disrupted by targeted homologous recombination. The mice have no detectable PTP-1B protein, yet appear to be physiologically normal. However, in the fed state on a normal diet, the mice have half the level of circulating insulin as their wild-type littermates. In glucose and insulin tolerance tests, the mice show an increased insulin sensitivity. When fed a high fat, high carbohydrate diet, the mice show a resistance to weight gain as compared to their wild-type littermates. Methods of making the mice and cell lines derived from the mice are also provided. The present invention also provides methods of identifying inhibitors of the enzymatic activity of PTP-1B as well as inhibitors identified by such methods.
Owner:TREMBLAY MICHEL +2
Lycium ruthenicum anthocyanin and preparation method thereof
PendingCN110498785AGood ability to scavenge DPPH free radicalsOrganic chemistryMetabolism disorderDPPHInsulin tolerance
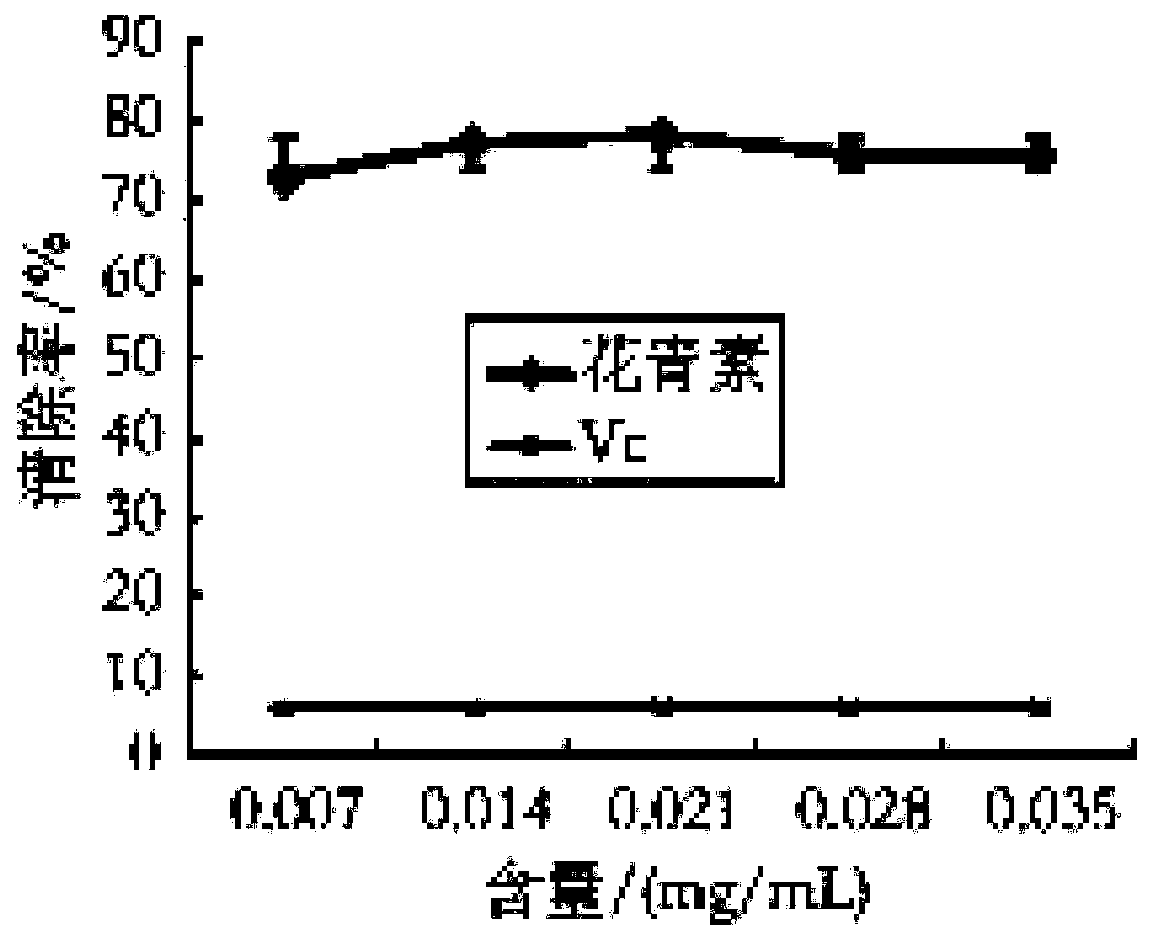
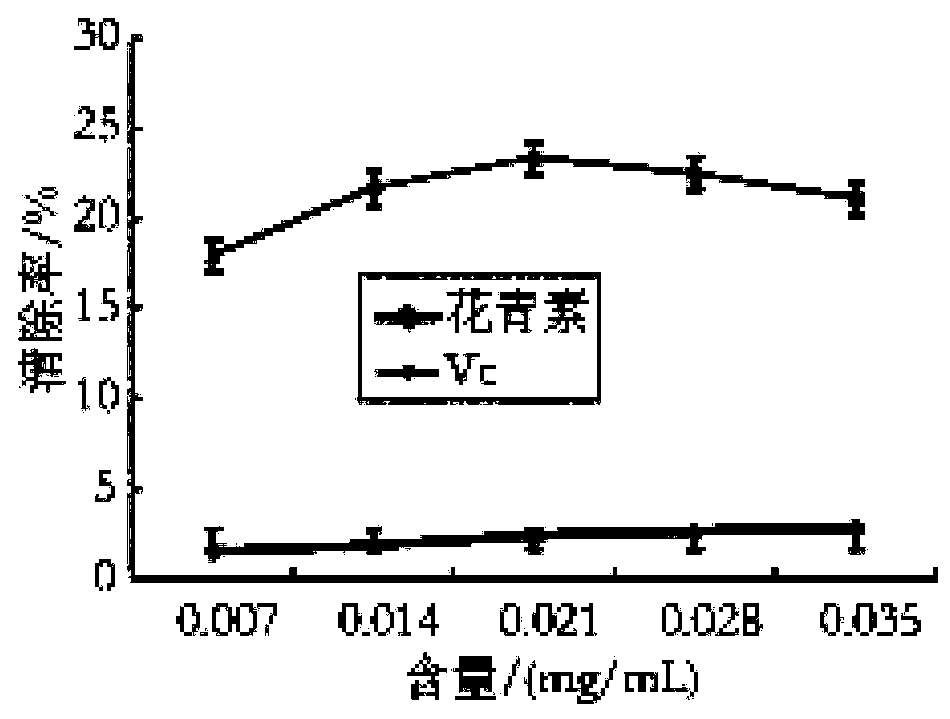
The invention discloses a Lycium ruthenicum anthocyanin and a preparation method thereof. The preparation method comprises the following steps: preparing a Lycium ruthenicum anthocyanin powder; extracting the Lycium ruthenicum anthocyanin; and purifying and freeze-drying the Lycium ruthenicum anthocyanin. The Lycium ruthenicum anthocyanin prepared in the invention has good ability to remove DPPH free radicals and hydroxyl free radicals, and has influences on fat accumulation, lipid metabolism and insulin tolerance of obese mice.
Owner:青海省第四人民医院
Ursine fat injection emulsion, and its prepn. method
InactiveCN1757396AImprove bioavailabilityMeet energy input requirementsMetabolism disorderMammal material medical ingredientsUltrasonic emulsificationInsulin tolerance
An emulsified injection of fur seal fat suitable for the patients of carbohydate metabolism dis-function and insulin tolerance is proportionally prepared from the purified fur seal fat, emulsifier, isotonic regulator, anti-oxidizing agent and the water for injection through ultrasonic emulsifying.
Owner:中国生化制药工业协会
Application of hydrogen sulfide and donor thereof sodium hydrosulfide to preparation of medicament for treating diabetes
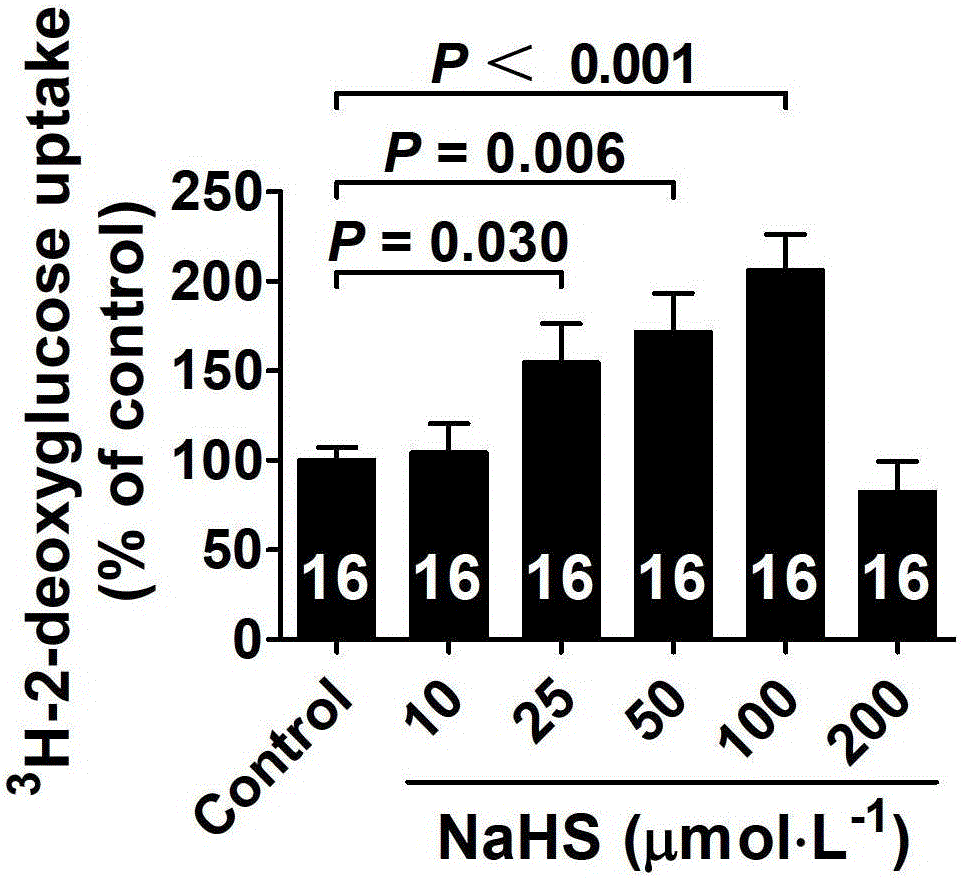
ActiveCN103622992AImprove the level ofProtectiveMetabolism disorderUrinary disorderSodium hydrosulfideGlucose uptake
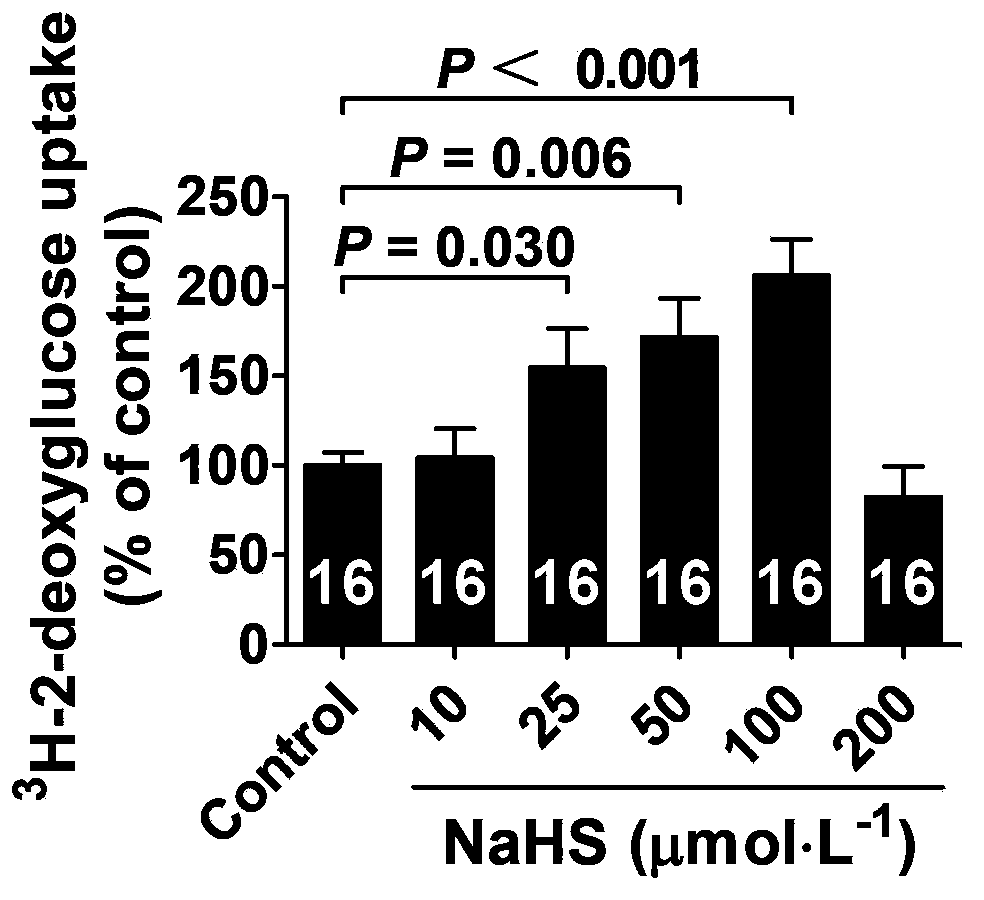
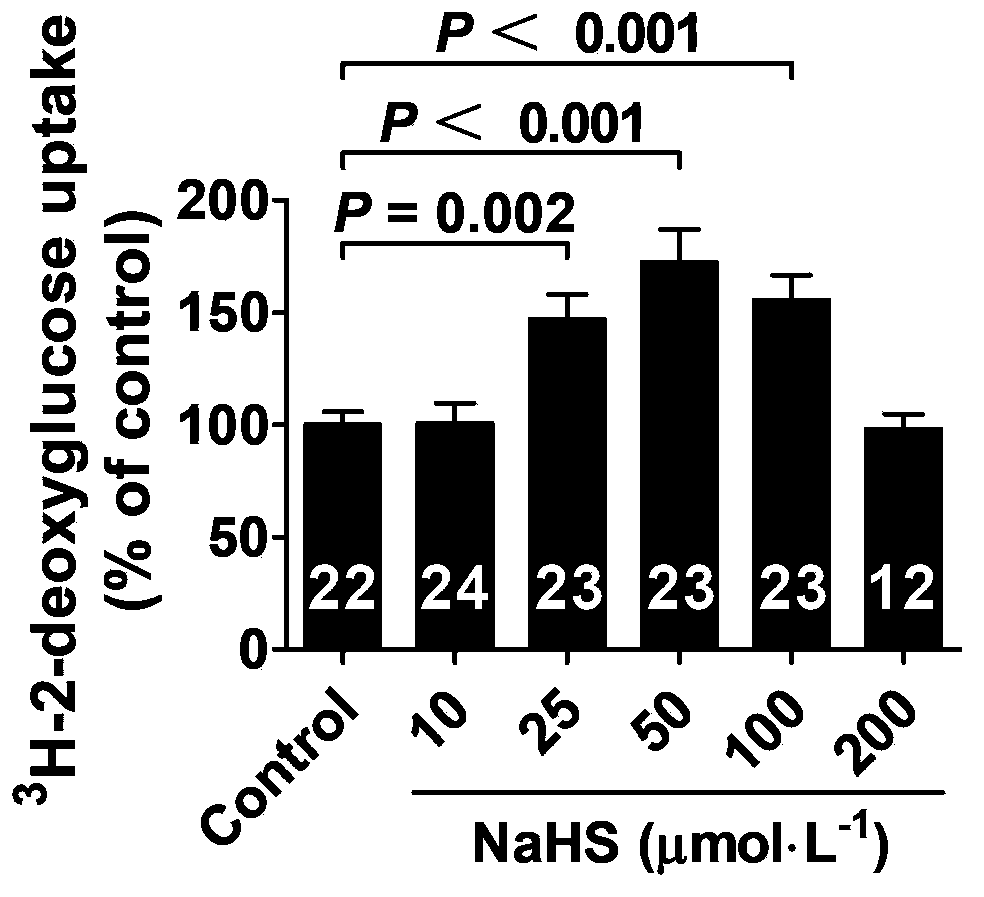
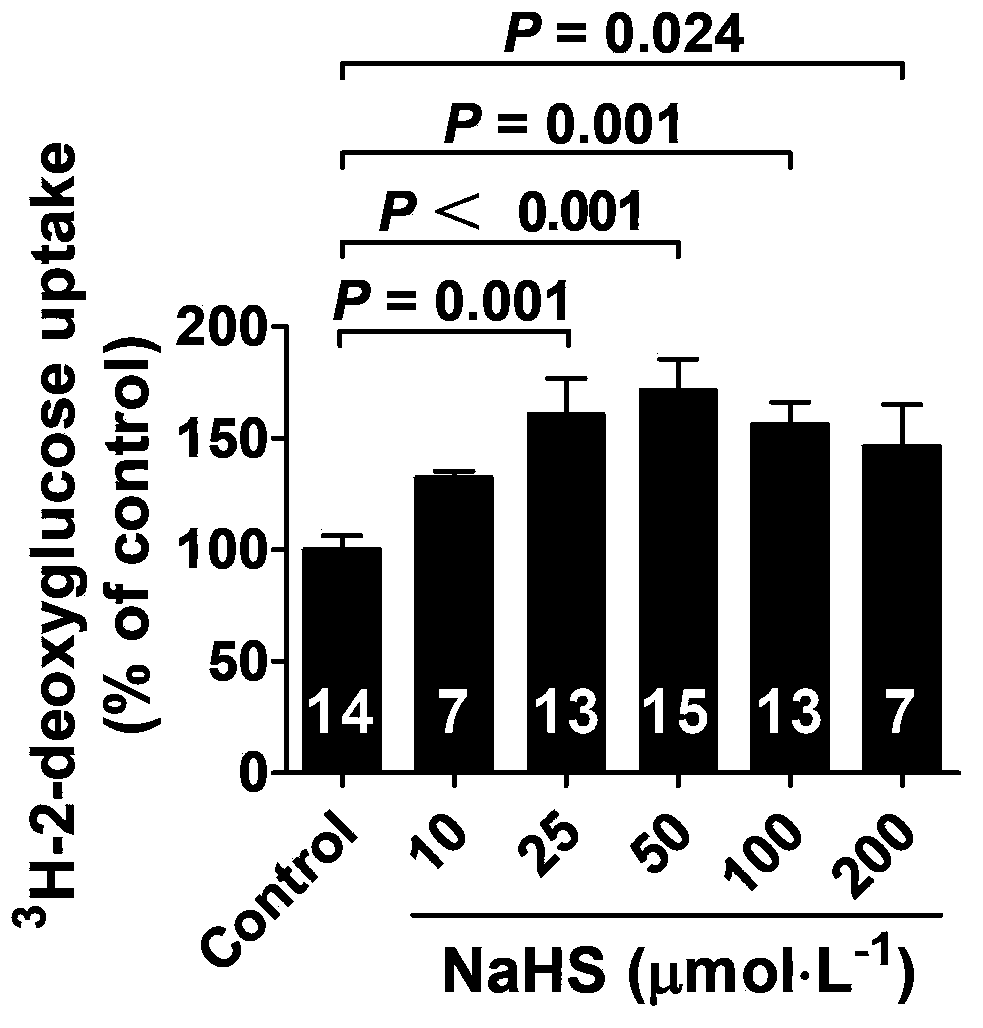
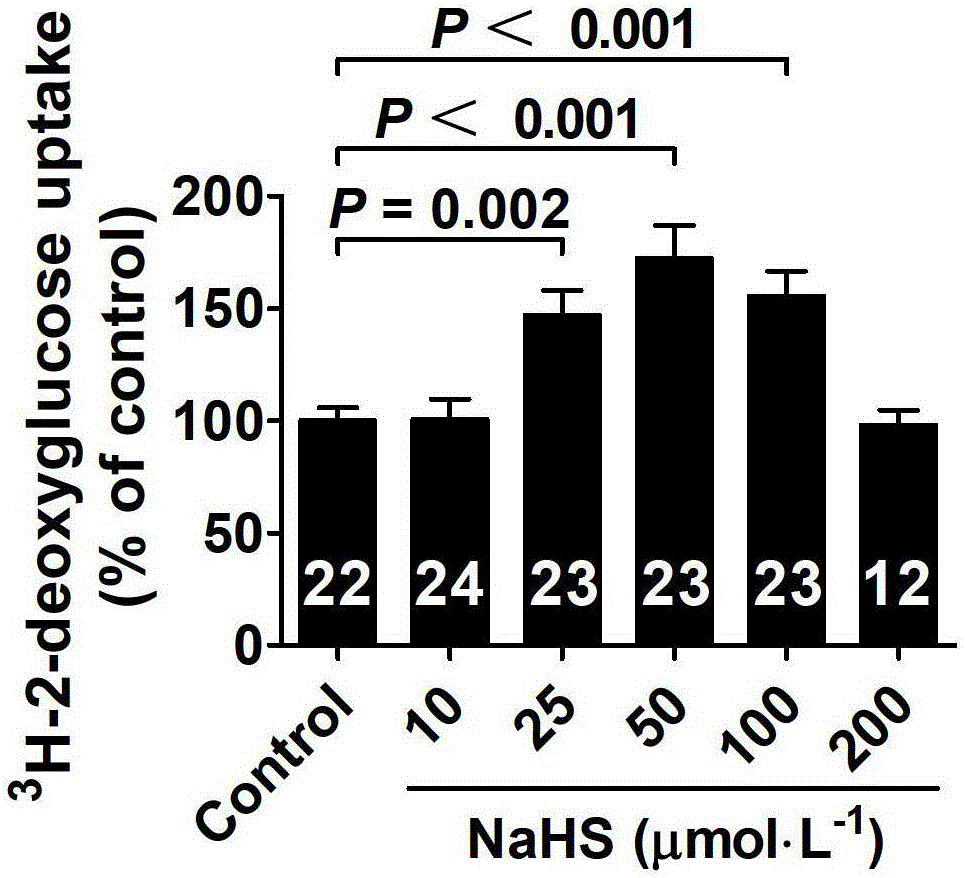
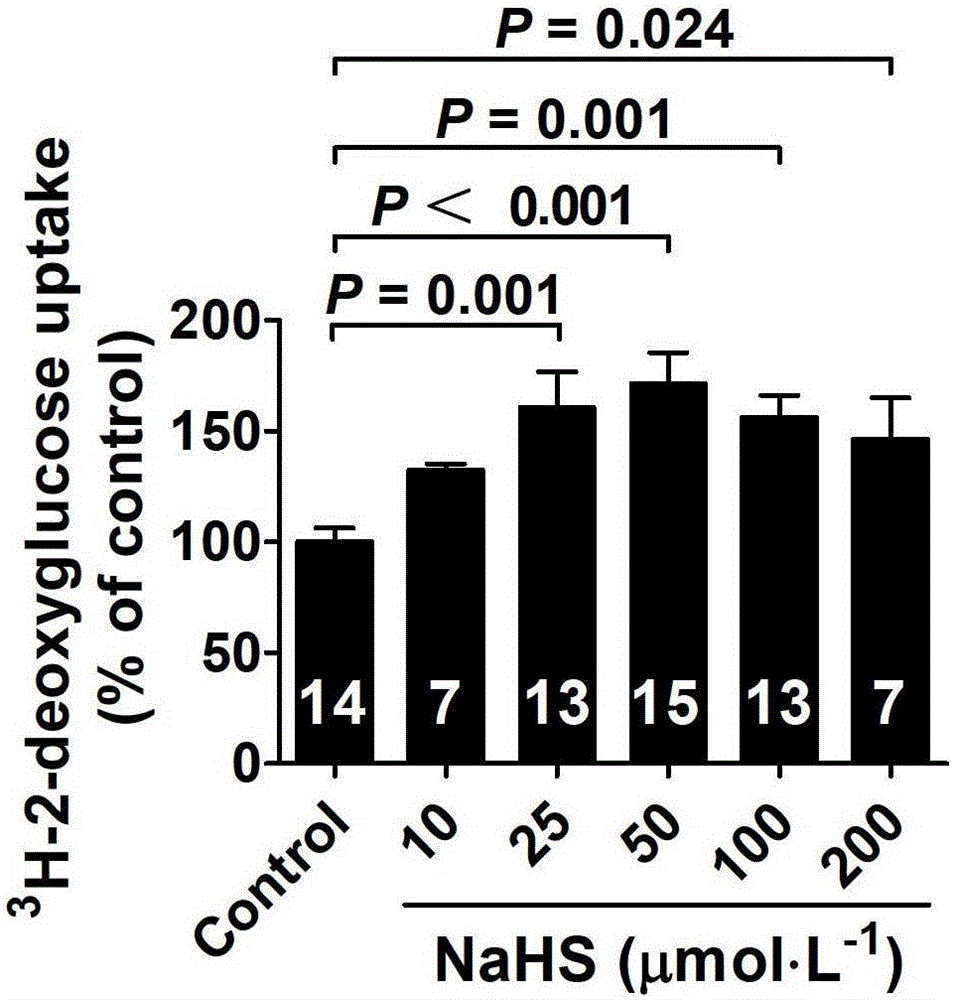
The invention belongs to the field of pharmacy, relates to novel medicinal application of hydrogen sulfide and a donor thereof sodium hydrosulfide to pharmacy, and in particular relates to application of hydrogen sulfide and donor thereof sodium hydrosulfide to preparation of medicament for treating diabetes. The invention adopts the exogenous hydrogen sulfide and its donor sodium hydrosulfide (NaHS) for insulin sensibilization and tests for regulating blood sugar level and increasing insulin level for type II diabetes. Through correlation test of skeletal muscle and adipose cells with insulin resistance, insulin tolerance test by using animal model for diabetes insulin resistance, glucose consumption experiment, animal model experiment for diabetes insulin resistance and NaHS intervention animal experiment, the results show that NaHS can promote glucose uptake in the presence of insulin, thus indicating insulin enhancement effect. The invention of hydrogen sulfide and the donor thereof sodium hydrosulfide can be used as insulin sensitizers and medicaments for regulating blood sugar level and increasing insulin level for type II diabetes.
Owner:FUDAN UNIV
Heterocyclic analog of diphenylethlene compound and application thereof
The invention discloses a novel compound formed by the chemical coupling of a diphenylethlene compound and derivatives thereof as well as thiazolidine or oxazolidine midbody and a preparation method thereof. The invention also discloses a plurality of pharmaceutical applications of the compounds: reducing blood sugar, serum insulin level and triglycercide level of a diabete II animal model, treating diseases related to the insulin tolerance, such as polycystic ovary syndrome, hyperlipemia, coronary disease and ambient vascular diseases, and also treating inflammations and immunological diseases, especially diseases mediated by cytokines and cyclooxygenase cox, such as THF-Alpha, IL-1, IL-6 and / or COX-2.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Lipid-lowering polypeptide and pharmaceutical application thereof
PendingCN111138552AImprove pathological conditionsStrong medicinePeptide/protein ingredientsMetabolism disorderObesity preventionFat mouse
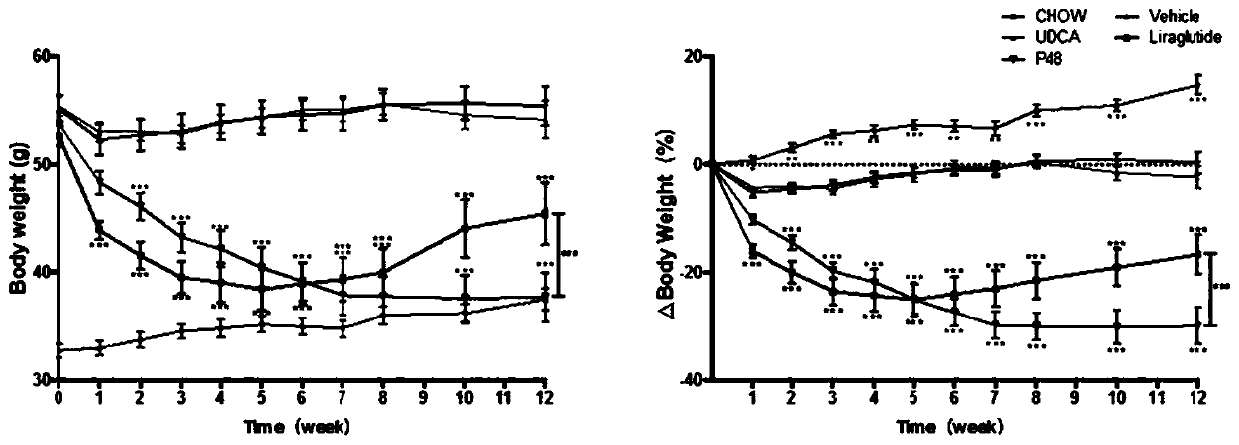
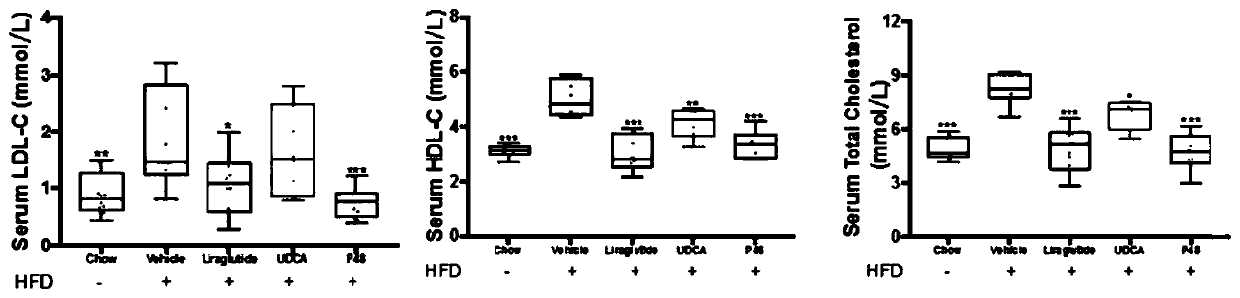
The invention designs a novel lipid-lowering polypeptide P48. The novel lipid-lowering polypeptide P48 is prolonged in a half-life period, plays the role of a GLP-1 receptor agonist and also plays therole of a GLP-1 analogue to adjust the fat content of an individual. The P48 peptide can be used for inhibiting feed intake of a non-alcoholic fatty liver model mouse induced by high-fat diet, remarkably reduce the weight of an obese mouse, lower the blood fat level of the obese mouse, maintain the normal form of the liver, inhibit liver cell injury, reduce accumulation of lipid in the liver andvacuolar deformation of cells, increase the level of adiponectin leptin in blood, and give a play to the blood glucose reducing and blood fat regulating effects of the adiponectin leptin; meanwhile, the P48 peptide obviously improves insulin tolerance and sugar tolerance. The novel lipid-lowering polypeptide P48 of the invention has potential pharmaceutical application in preparation of drugs fortreating and preventing obesity or complications thereof, namely non-alcoholic fatty liver diseases.
Owner:CHINA PHARM UNIV
Composition for body fat reduction
InactiveCN101111239ASenses disorderNervous disorderHaematococcus pluvialis extractAdditive ingredient
An increase in body fat is one of main causes of life-style-related diseases such as hypertension, insulin tolerance, and hyperlipemia. A composition for body fat reduction is provided which inhibits body fat from decreasing or increasing and which is effective in the treatment and prevention of adult diseases a cause of which is body fat. Also provided are a medicine and a food each containing the composition. [MEANS FOR SOLVING PROBLEMS] The composition for body fat reduction comprises (A) astaxanthin and (B) at least one of an active ingredient, an adsorbent, and a solubilizer. Alternatively, it comprises (A) a Haematococcus pluvialis extract containing astaxanthin and (B) at least one of an active ingredient, an adsorbent, and a solubilizer.
Owner:FUJI CHEM IND CO LTD
Method for constructing insulin resistance animal model
InactiveCN102228700AModeling method is simpleHigh molding ratePeptide/protein ingredientsBiological testingPhysiologyStatistical analysis
The invention relates to a method for constructing an insulin resistance animal model. A Sprague Dawley rat is injected with glucose oxidase (400U / kg weight) through tail vein injection to induce to generate insulin resistance. The statistical analysis of ghlcose tolerance and insulin tolerance and the detection of insulin signal transduction prove that the glucose oxidase constructed insulin resistance animal model is feasible, and can serve as a research model for insulin resistance, the pathogenesis of type 2 diabetes and a medicine function mechanism. Compared with the conventional model constructing technology, the method has the advantages of simple modeling method, high modeling efficiency, low cost, short period, high repeatability and the like.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Medical application of genistein chromium complex to treatment of diabetes
ActiveCN105106221ALower blood sugarImproves glucose tolerance levelOrganic active ingredientsMetabolism disorderFasting glucosePancreatic hormone
The invention provides medical application of a genistein chromium complex to treatment of diabetes. Results of the animal growth condition research, fasting blood-glucose value determination, glucose tolerance test, insulin tolerance test show that the genistein chromium complex has an excellent hypoglycemic effect, and can be used for preparing drugs for treating diabetes II; by combing the hypoglycemic advantage of chromium, the hypoglycemic activity of genistein is improved obviously and toxicity of inorganic chromium can be reduced.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Method for establishing insulin resistance animal model
InactiveCN109430156AAvoid the problems of instability and low molding rateGuaranteed stabilityAccessory food factorsGlycolipid metabolismBiology
The invention discloses a method for establishing an insulin resistance animal model. According to the method, feed with high content of selenium, sugar and fat and low-concentration fructose drinkingwater are used for inducing inbred line C57BL / 6 mice with highly similar genetic backgrounds in order to cause glycolipid metabolism disorder of the mice and then induce the occurrence of insulin resistance, and then the insulin resistance animal model is established. Through unique proportioning of the feed, a pathogenesis process of the insulin resistance caused by the change of a dietary structure is highly simulated, comparison between the induced animal model and a control group shows that animals in a model group are obviously different from animals in the normal control group in intakeglucose tolerance, insulin tolerance, animal insulin resistance index and insulin sensitivity index, and it is indicated that the animals in the model group have obvious insulin resistance. The method has the advantages that the special feed is adopted for feeding in two modes, adverse reactions caused by chemical reagents are eliminated, a molding method is simple, the molding rate is high, thecost is low, and the reproducibility is high.
Owner:浦江县欧立生物技术有限公司
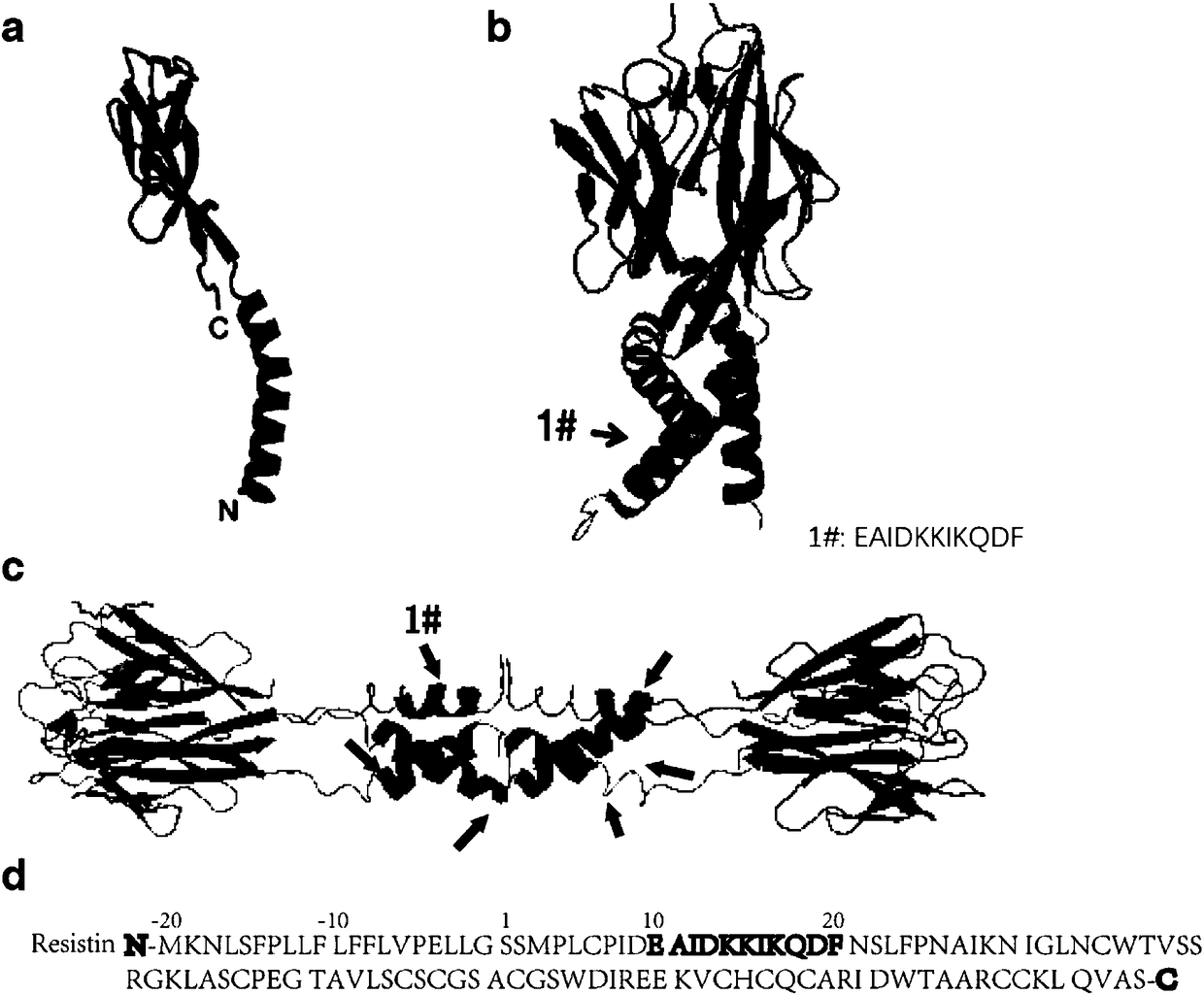
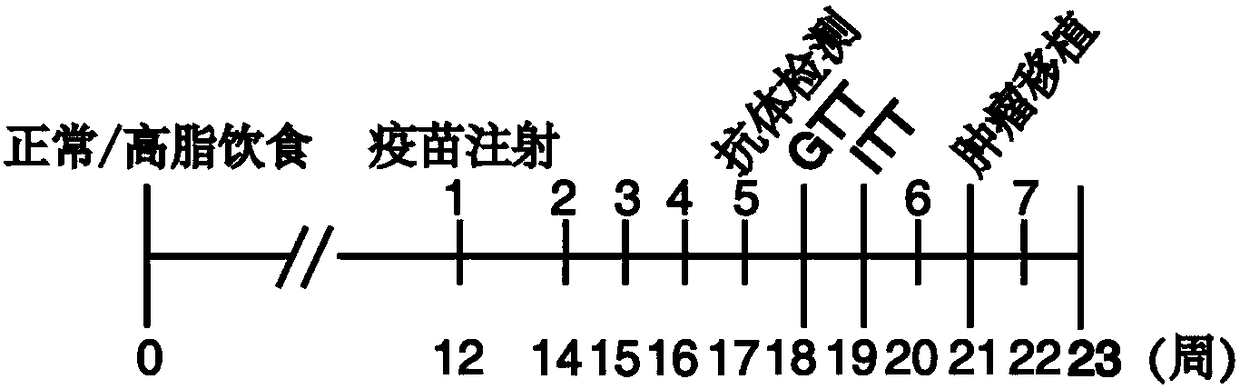
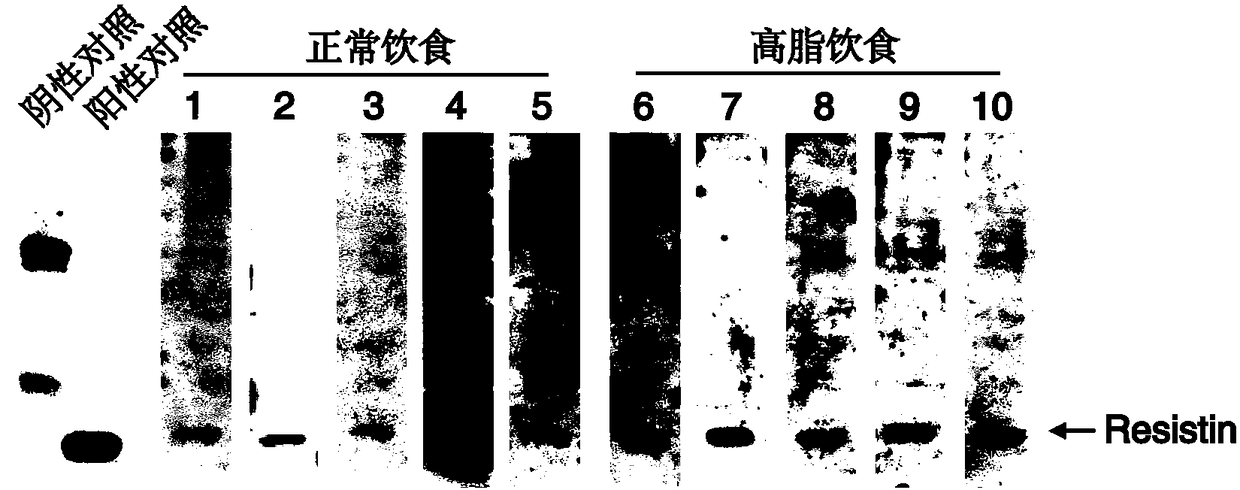
Resistin polypeptide vaccine and application thereof to treatment of mammary cancer
ActiveCN108379568AGrowth inhibitionPromote growthPeptide/protein ingredientsAntibody medical ingredientsSubcutaneous injectionLymphocyte
The invention discloses a resistin polypeptide vaccine and application thereof to the treatment of a mammary cancer, and belongs to the technical field of biology. A polypeptide vaccine provided by the invention is prepared from a Resistin-protein specific polypeptide segment and an immunologic adjuvant, and becomes the Resistin polypeptide vaccine. Through multiple subcutaneous injections, the vaccine can be used for activating the lymphocyte of an organism, and generates an immune neutralizing antibody aiming at Resistin protein. The Resistin polypeptide vaccine does not influence the sugartolerance and insulin tolerance levels of the organism at all. The Resistin polypeptide vaccine can be used for obviously inhibiting the growth of an ER (Estrogen Receptor) positive mammary tumor anda triple-negative mammary tumor under a mouse model; in addition, the Resistin polypeptide vaccine can be used for obviously inhabiting the growth of the ER positive mammary tumor and the triple-negative mammary tumor of a mouse under an obesity model induced by a high fat diet, and a new way is provided for ameliorating a current treatment method of the mammary cancer by the resistin polypeptidevaccine.
Owner:ACADEMY OF MILITARY MEDICAL SCI
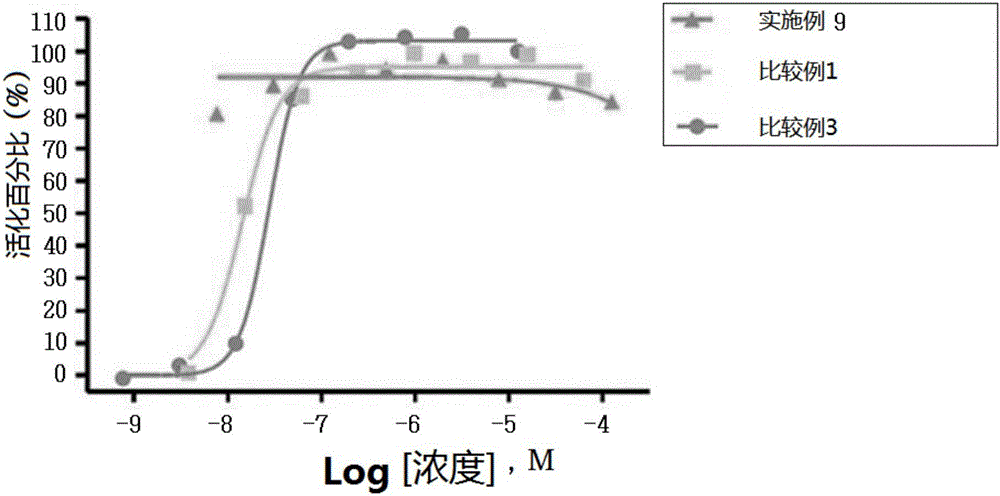
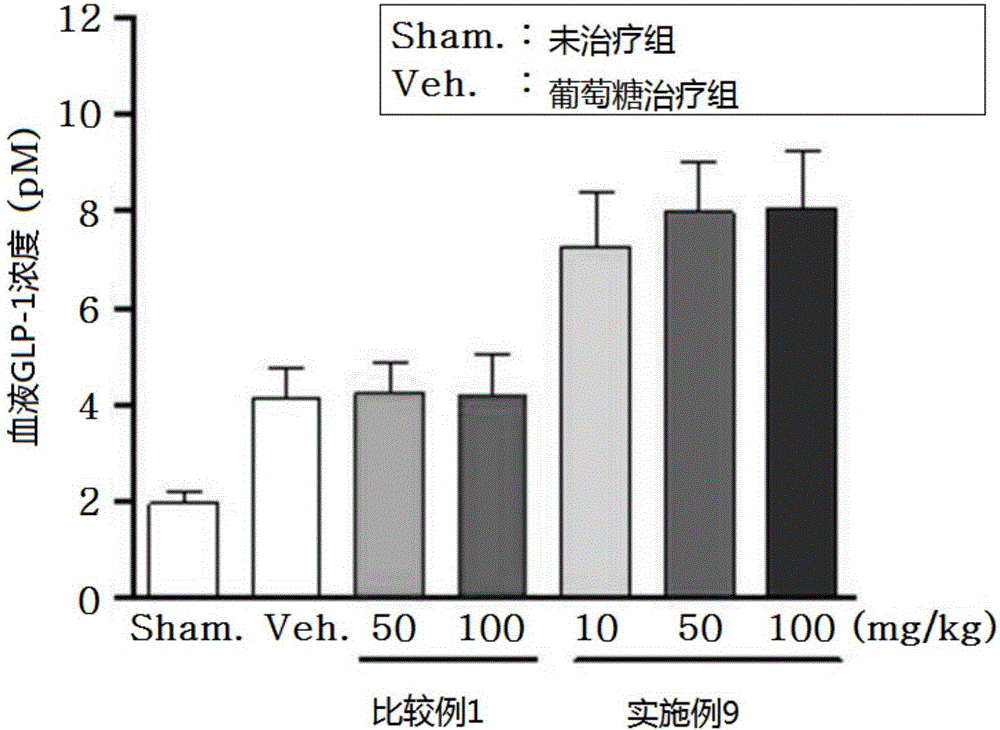
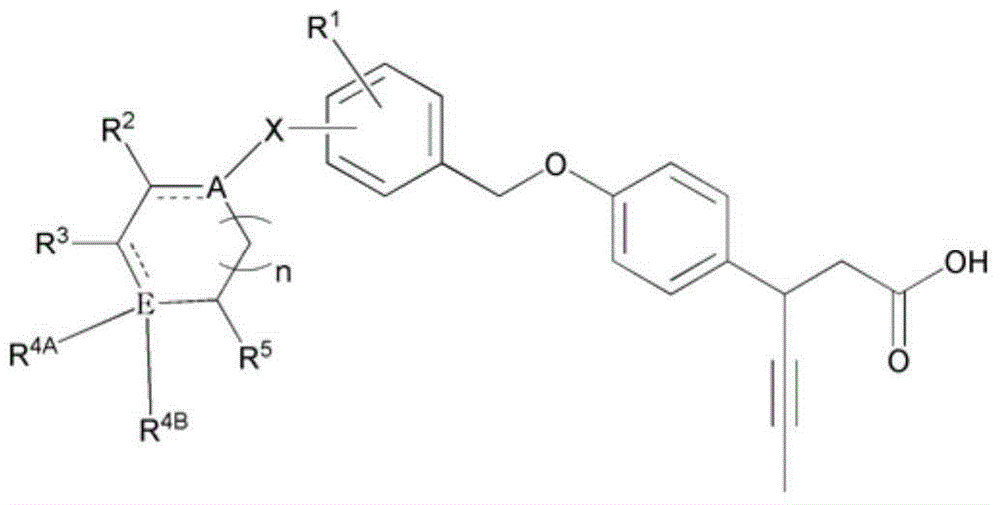
Novel 3-(4-(benzyloxy)phenyl)hex-4-inoic acid derivative, method of preparing same and pharmaceutical composition for preventing and treating metabolic disease including same as effective ingredient
ActiveCN105121423APromote activationPromote secretionOrganic compound preparationMetabolism disorderAcute hyperglycaemiaDyslipidemia
The present invention relates to a novel 3-(4-(benzyloxy)phenyl)hex-4-inoic acid derivative, a method of preparing same and a pharmaceutical composition for preventing and treating metabolic disease including same as an effective ingredient. The novel 3-(4-(benzyloxy)phenyl)hex-4-inoic acid derivative, an optical isomer thereof, or a pharmaceutically acceptable salt thereof, according to the present invention, has an excellent effect on activation of GPR40 protein and thus has excellent promoting effect of insulin secretion; is nontoxic when co-administrated with other drugs and is possibly co-administrated with other drugs; and has excellent effective effect of activating GPR40 protein in vivo. Therefore, a composition including the novel 3-(4-(benzyloxy)phenyl)hex-4-inoic acid derivative, the optical isomer thereof, or the pharmaceutically acceptable salt thereof may be availably used in a pharmaceutical composition for preventing and treating metabolic disease such as obesity, type I diabetes, type II diabetes, incompatible glucose tolerance, insulin tolerance, hyperglycemia, hyperlipidemia, hypertriglyceridemia, hypercholesterolemia, dyslipidemia, syndrome X, etc.
Owner:HYUNDAI PHARMA
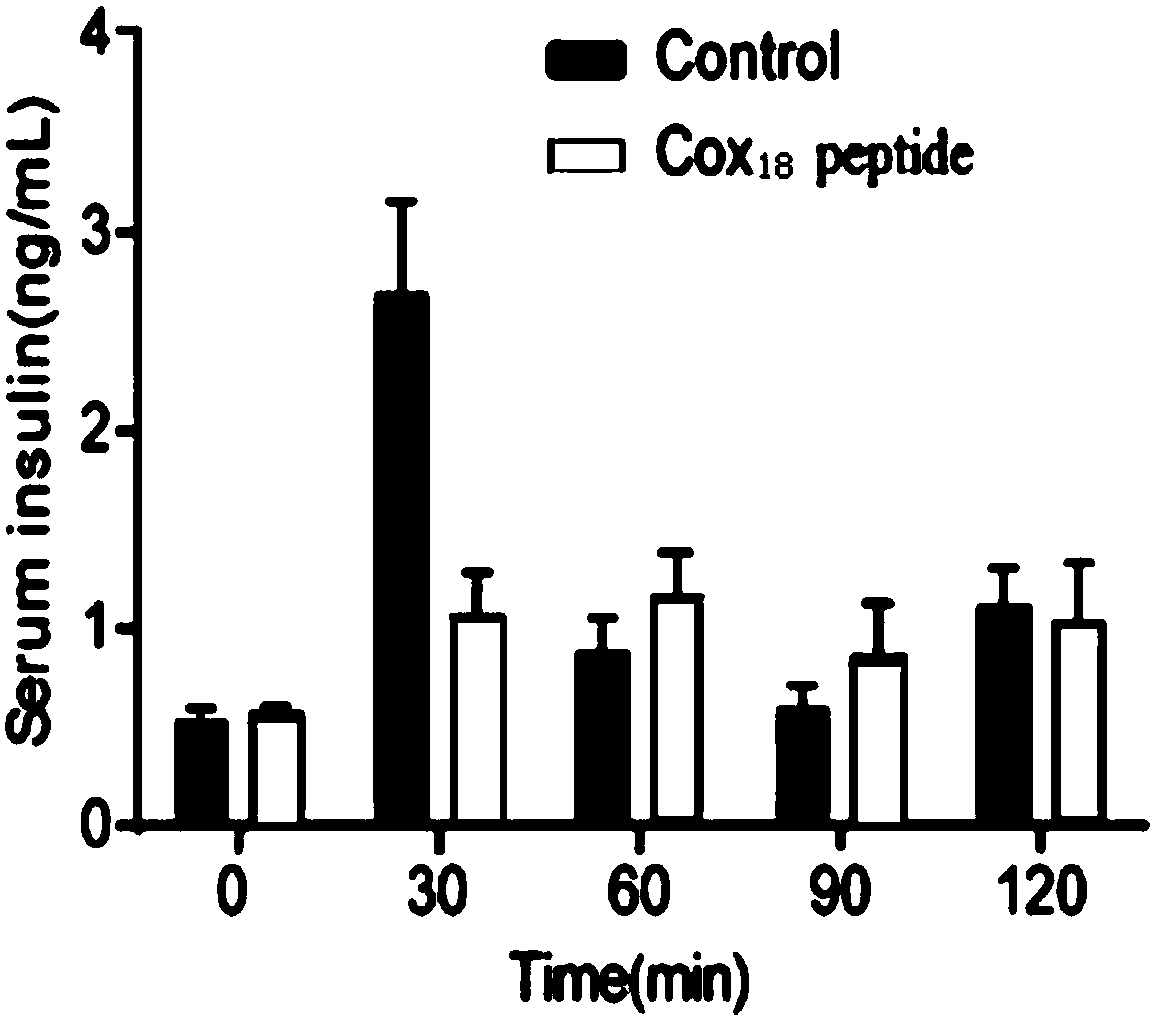
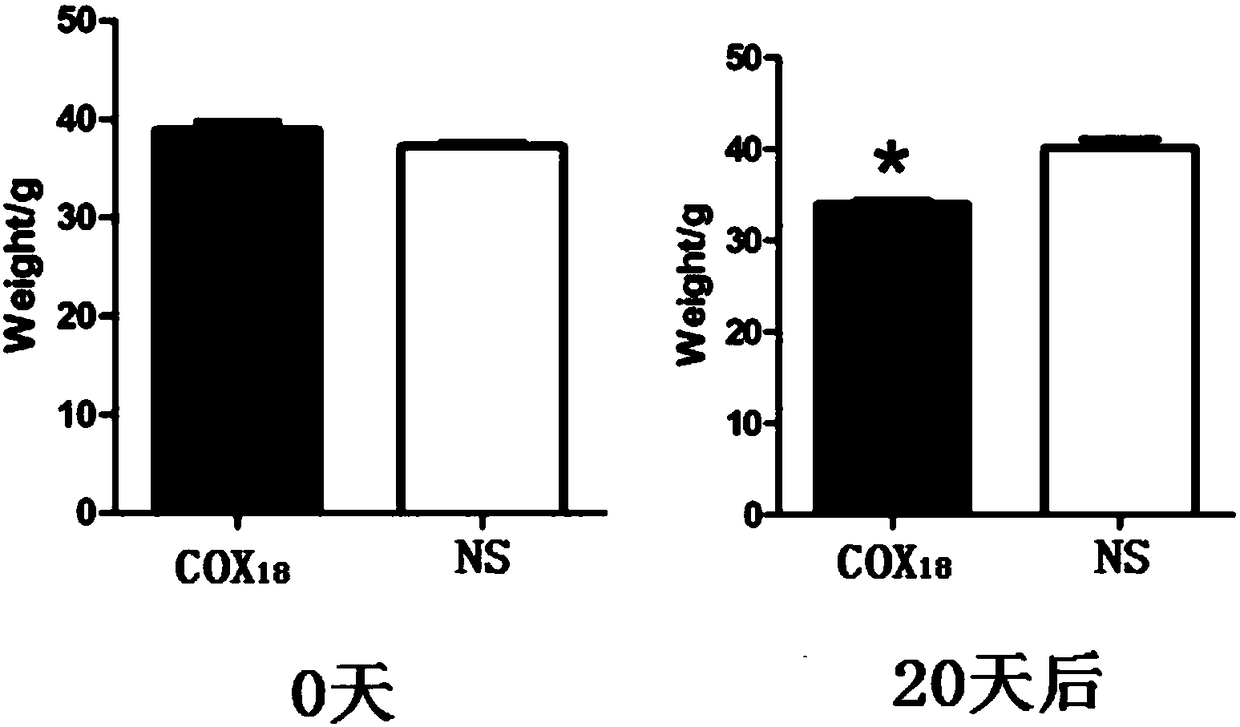
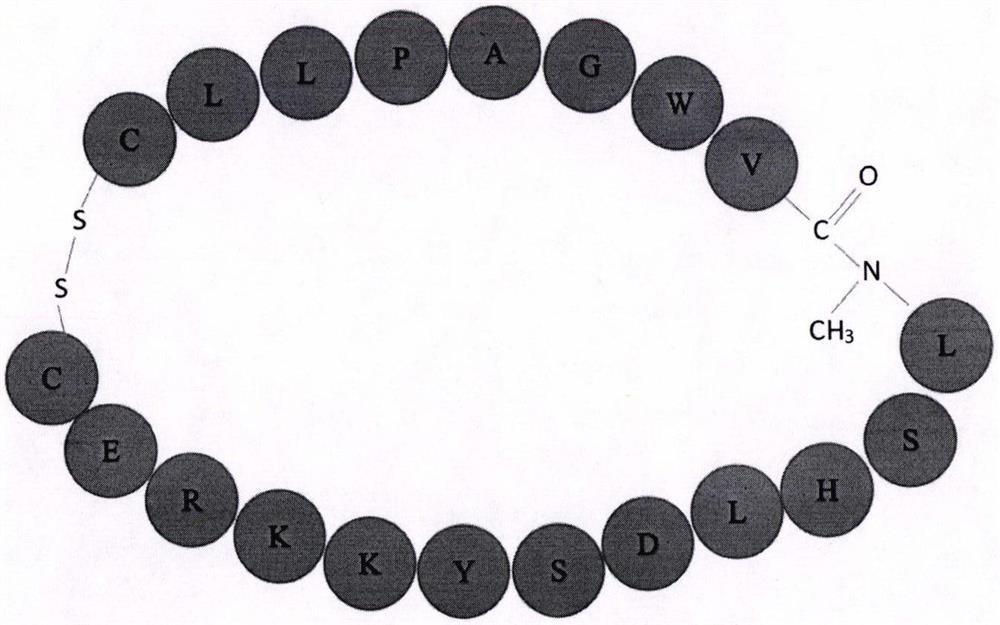
COX18 polypeptide with biological activity as well as synthesis method thereof and application thereof
InactiveCN108315308ASimplify biochemical tedious proceduresLow costPeptide/protein ingredientsMetabolism disorderChemical synthesisSide effect
The invention provides a COX18 polypeptide with biological activity. An amino acid sequence of the polypeptide is as shown in SEQ ID NO. 1: LLPAGWILSH LETYRRPE. The invention further provides a methodfor synthesizing the COX18 polypeptide through a chemical synthesis method, namely an Fmoc method as well as application, and the polypeptide is applicationd for preparing drugs which inhibit sugar induced insulin secretion, resist insulin tolerance, resist hyperinsulinemia and reduce body weight. The invention finds a novel COX18 polypeptide which is 52-69th part of human cytochrome c oxidase 8A, which is different from the precious porcine sequence. Compared with the previous sequence, the sequence has 38.88% of different amino acid. Moreover, the sequence is humanized, and has better homology, fewer immunoreactions and side effects while being applied to a human body.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Secretion-type FNDC5 protein and preparation method and application thereof
ActiveCN110684100AImprove enduranceImprove the immunityHormone peptidesPeptide/protein ingredientsFat mousePancreatic hormone
The invention discloses a secretion-type FNDC5 protein. The secretion-type FNDC5 protein does not contain a transmembrane domain and can be directly secreted and released into blood; and the amino acid sequence of the FNDC5 protein is SEQ ID NO:1. The secretion-type FNDC5 protein is simple in structure and convenient to obtain. The secretion-type FNDC5 protein can be used for treating obesity-related metabolic disorders; after the secretion-type FNDC5 recombinant protein is injected into the abdominal cavity of a high-fat fed obese mouse model, glucose and insulin tolerance can be effectivelyimproved, and it shows that the protein can obviously relieve glucose metabolic disorders and insulin resistance of obese patients; and fatty liver occurrence can be obviously relieved, the level of plasma cholesterol, free fatty acid and triglyceride is decreased, and it shows that the protein can relieve the lipometabolic disorders of the obese patients. The secretion-type FNDC5 protein can serve as a new drug target for treating the metabolic disease to be developed.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
Application and construction method of high-selenium induced insulin resistance animal model
InactiveCN103211834AHigh molding rateModeling method is simpleSulfur/selenium/tellurium inorganic active ingredientsRat modelInsulin humulin
The invention discloses an application and construction method of a high-selenium induced insulin resistance animal model. The application and construction method comprises the following steps of: feeding 100mug / kg-200ug / kg of selenium to the stomach of an SD (Sprague Dawley) rat every day; and six weeks later, obtaining the insulin resistance animal model. According to the statistic analysis of glucose tolerance and insulin tolerance, insulin signal transduction and gluconeogenesis key enzyme test, the construction of the insulin resistance animal model by utilizing sodium selenite is feasible; and the insulin resistance animal model can be used as a high-selenium induced insulin resistance animal model and a pathogenesis and drug action mechanism research model of diabetes mellitus II. The insulin resistance rat model constructed by the application and the construction method disclosed by the invention has the advantages of simpleness in modeling method, high model establishing success rate, low cost, short period, good reproducibility and the like.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Antibodies that recognize iapp
InactiveUS20150110776A1Reduce decreaseReducing IAPP accumulationSugar derivativesBacteriaIGT - Impaired glucose toleranceEpitope
The invention provides monoclonal antibody 3H6 and related antibodies. The 3H6 antibody binds to an epitope within residues 28-36 of IAPP. The antibodies of the invention are useful, for example, for treating disorders associated with IAPP accumulation, particularly accumulation of IAPP deposits. Such disorders include type 2 diabetes, metabolic syndrome, impaired insulin tolerance, impaired glucose tolerance, insulinomas, and related conditions.
Owner:PROTHENA BIOSCI LTD
Application and construction method of animal model of insulin resistance induced by high selenium
InactiveCN103211834BHigh molding rateModeling method is simpleSulfur/selenium/tellurium inorganic active ingredientsRat modelInsulin humulin
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Antibodies that recognize iapp
InactiveUS20150110777A1Reduce decreaseReducing IAPP accumulationAnimal cellsBacteriaEpitopeIGT - Impaired glucose tolerance
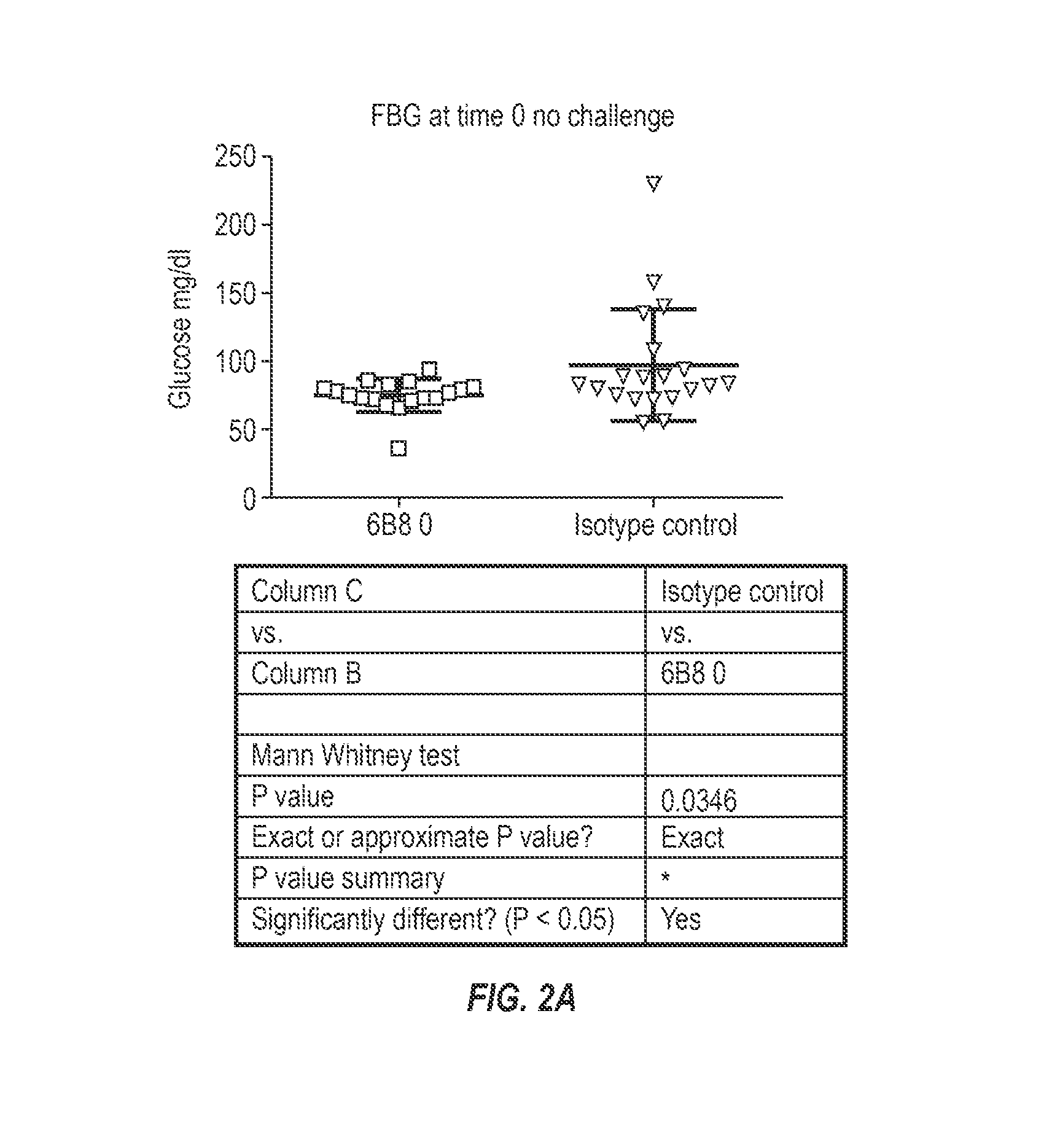
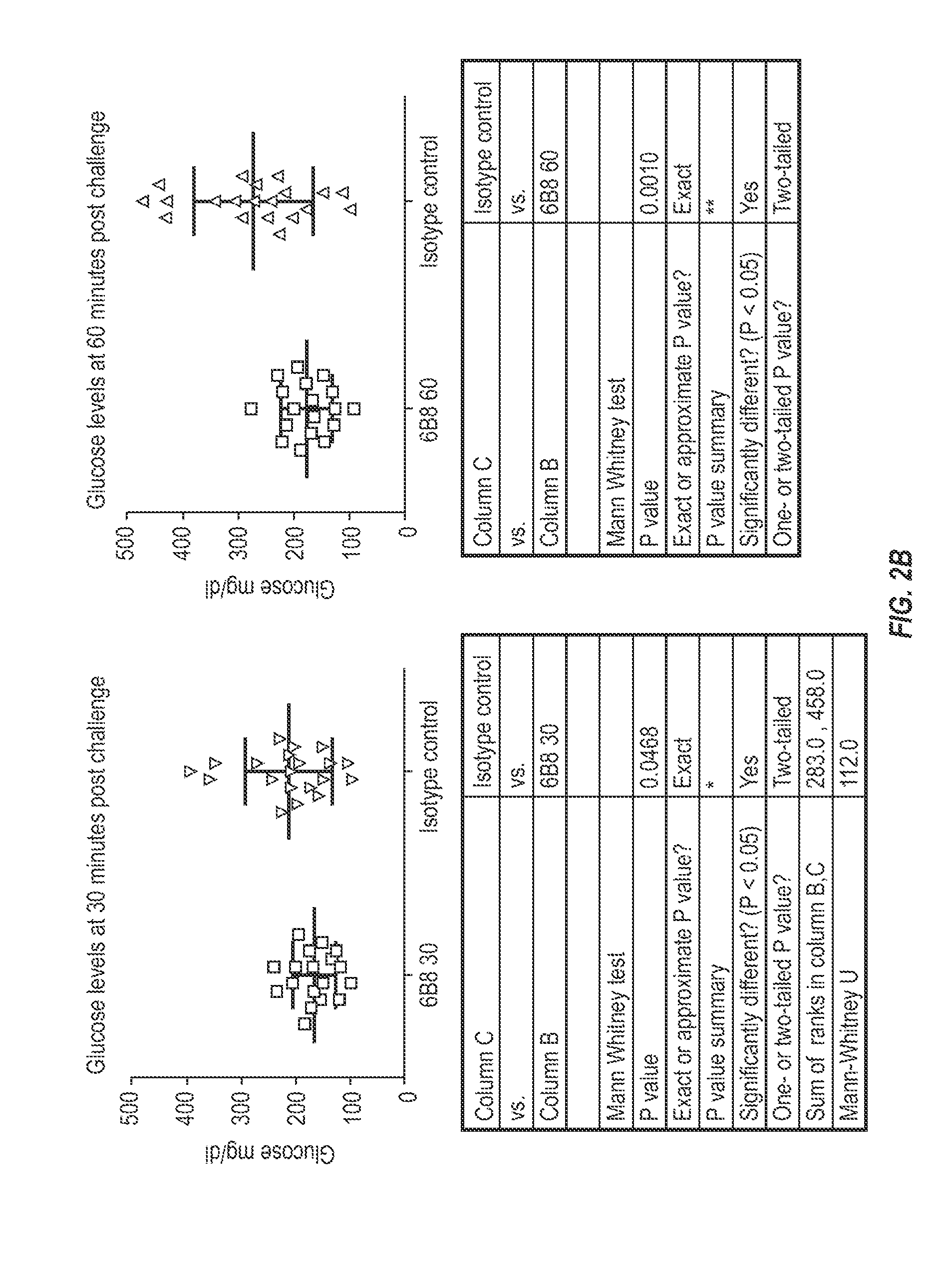
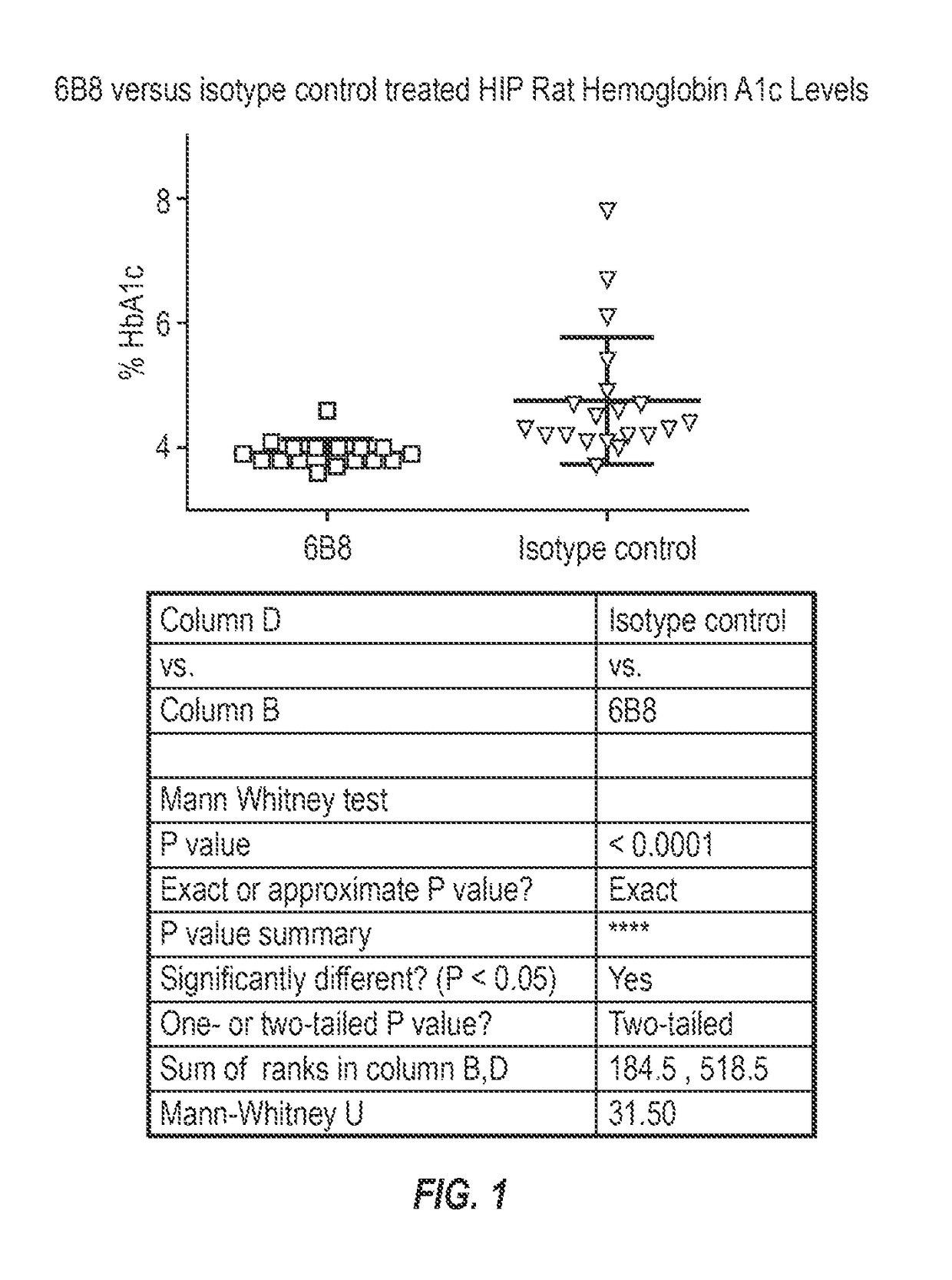
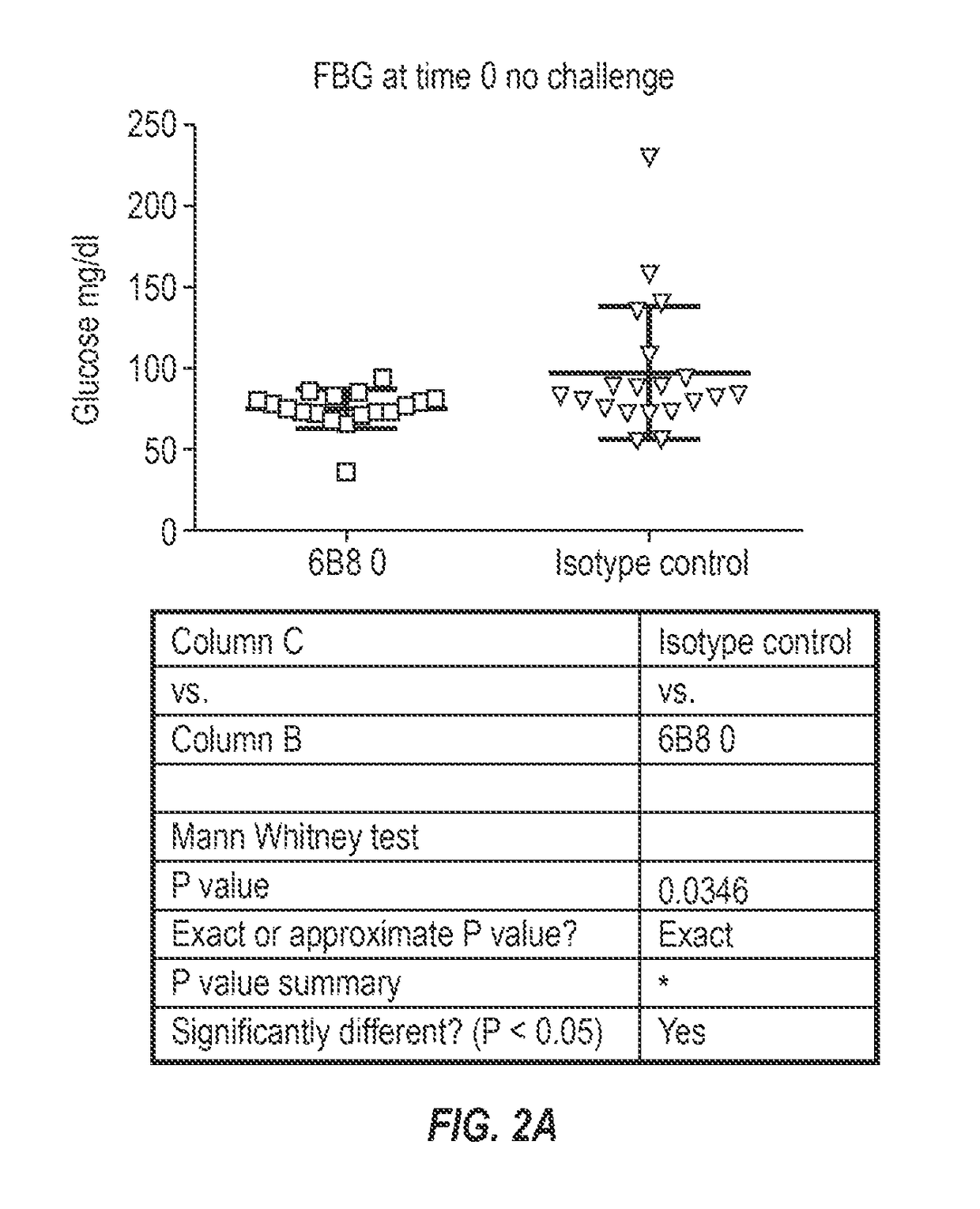
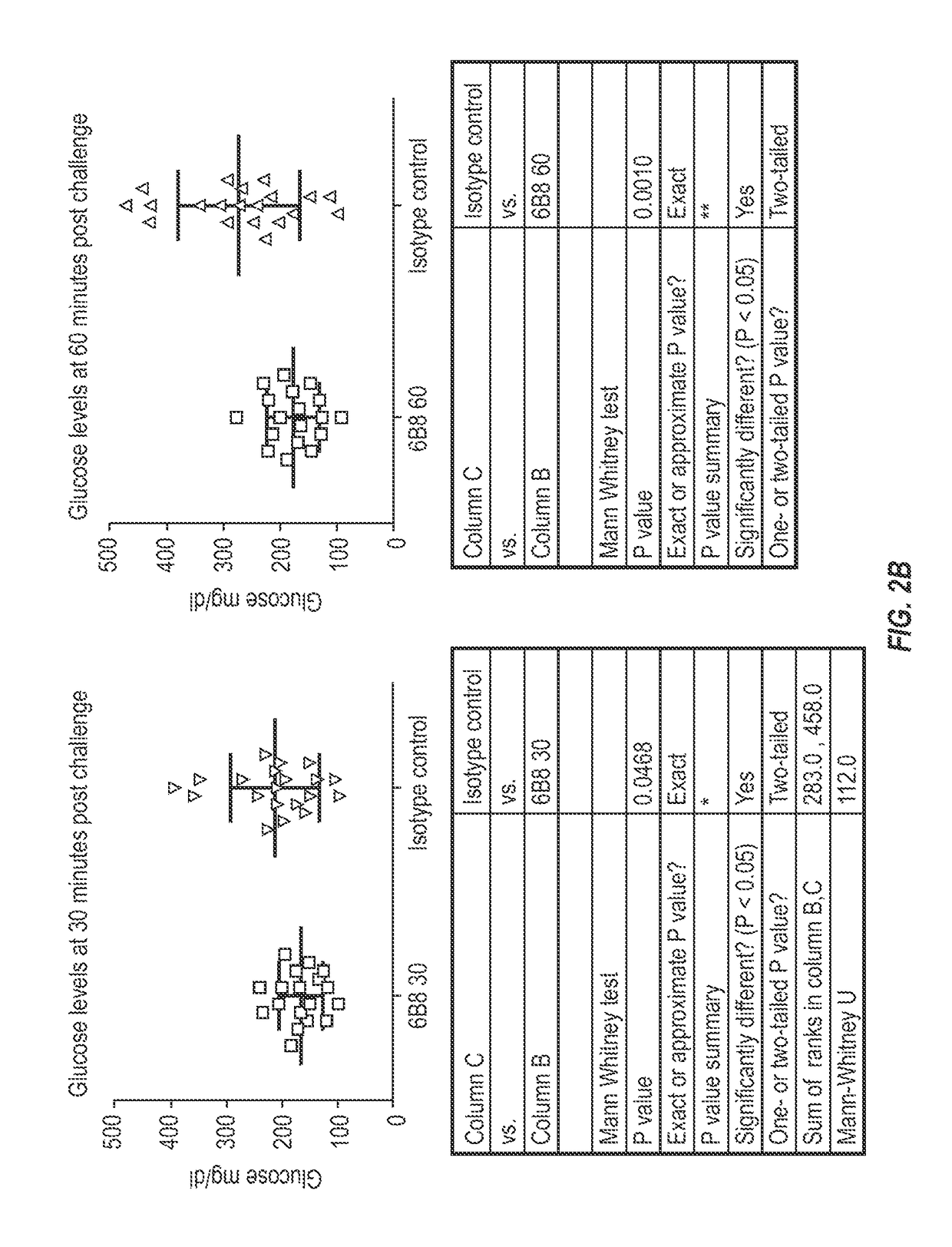
The invention provides monoclonal antibody 6B8 and related antibodies. The 6B8 antibody binds to an epitope within residues 3-12 of IAPP. The antibodies of the invention are useful, for example, for treating disorders associated with IAPP accumulation, particularly accumulation of IAPP deposits. Such disorders include type 2 diabetes, metabolic syndrome, impaired insulin tolerance, impaired glucose tolerance, insulinomas, and related conditions.
Owner:PROTHENA BIOSCI LTD
Method for building small intestine insulin expression reduction rat model by adenovirus vector
InactiveCN108795984ASuccessful and reliableEasy to makeFermentationDsDNA virusesGeneticsInsulin tolerance
The invention discloses a method for building a small intestine insulin expression reduction rat model by an adenovirus vector. The method mainly comprises the steps of adenovirus vector building andrat small intestine transfection by the adenovirus vector. The characteristic that the adenovirus vector carries rat antisense mRNA insulin gene is utilized; through rat gavage, the rat small intestine insulin expression reduction can be built within three months; the reduction of rat insulin tolerance and oral glucose tolerance can be caused. The animal model has wide application prospects for further developing medicine for treating diabetes.
Owner:TIANJIN MEDICAL UNIV
Novel pharmaceutical composition for diabetes prevention and treatment
InactiveCN103768061AMaintenance activationExcellent tolerance improvementOrganic active ingredientsMetabolism disorderSide effectTolerability
The invention aims to research a combined treatment of pioglitazone and rosiglitazone, and relate to a novel pharmaceutical composition for diabetes prevention and treatment which has an excellent function of PPAR excitation (activation) and which alleviate side effects at the same time. The PPAR excitation function can be maintained by giving the pioglitazone and rosiglitazone, preparations of acceptable slat thereof in the pharmacology, or alternatively giving the abovementioned single dosages and the acceptable slat thereof in the pharmacology. The novel pharmaceutical composition has an excellent insulin resistance function and an excellent hypoglycemic effect, and can alleviate side effects when being used as a single dosage.
Owner:王松
Application of cationic polymer in medicine preparation
PendingCN114146184ADecreased blood flowRecovery functionAntibacterial agentsOrganic active ingredientsIntestinal microorganismsPancreatic hormone
The invention relates to application of a cationic polymer in preparation of a medicine for preventing or treating diseases caused by toxins generated by intestinal microorganisms or diseases caused by new coronavirus infection. Toxin released by intestinal microorganisms can be neutralized and cleared by oral administration of cationic polymers, and most of these microbial toxins present anionic acidity. By adopting the application, various intestinal microbial toxins entering blood can be reduced; therefore, systemic inflammation, local inflammation, hepatic failure and liver injury, insulin tolerance, fatty liver disease, lung failure, kidney failure, hepatic encephalopathy, septicemia and inflammatory storm, severe illness and the like caused by novel coronavirus infection are relieved; the traditional Chinese medicine composition can also be used for treating ICU patients, reducing systemic inflammation, recovering organ functions and improving the survival rate, and can also be used for preventing or treating chronic inflammation and various metabolic diseases caused by intestinal bacterial toxins, preventing and treating fatty liver diseases, relieving insulin tolerance, relieving diabetes mellitus and relieving obesity.
Owner:SQ BIOPHARMA INC
Antibodies that recognize IAPP
InactiveUS9850302B2Reducing IAPP accumulationReduce accumulationMetabolism disorderAntibody ingredientsIGT - Impaired glucose toleranceEpitope
The invention provides monoclonal antibody 6B8 and related antibodies. The 6B8 antibody binds to an epitope within residues 3-12 of IAPP. The antibodies of the invention are useful, for example, for treating disorders associated with IAPP accumulation, particularly accumulation of IAPP deposits. Such disorders include type 2 diabetes, metabolic syndrome, impaired insulin tolerance, impaired glucose tolerance, insulinomas, and related conditions.
Owner:PROTHENA BIOSCI LTD
Use of hydrogen sulfide and its donor sodium hydrosulfide in the preparation of medicines for treating diabetes
ActiveCN103622992BImprove the level ofProtectiveMetabolism disorderUrinary disorderElevated insulinElevated insulin level
The invention belongs to the field of medicine and pharmacy, and relates to the new medical application of hydrogen sulfide and its donor sodium hydrosulfide in pharmacy, in particular to the application of hydrogen sulfide and its donor sodium hydrosulfide in preparing medicines for treating diabetes. The present invention uses exogenous hydrogen sulfide and its donor sodium hydrogen sulfide NaHS for insulin sensitization and tests for lowering blood sugar and increasing insulin levels for type II diabetes. Through correlation tests between skeletal muscle and adipocytes and insulin resistance, diabetic insulin The animal model of resistance conducts insulin tolerance test, glucose consumption test, animal model test of diabetes insulin resistance, and NaHS intervention animal experiment, etc. The results show that: NaHS can play a role in promoting glucose uptake in the presence of insulin, showing enhanced Insulin action. The hydrogen sulfide and its donor sodium hydrosulfide can be used as an insulin sensitizer and a drug for lowering blood sugar and increasing insulin level for type II diabetes.
Owner:FUDAN UNIV
Pharmaceutical composition containing pioglitazone and glimepiride
InactiveCN104288161AEnhanced inhibitory effectReduce inhibitionMetabolism disorderSulfonylurea active ingredientsGlucose utilizationInsulin dependent
The invention provides a pharmaceutical composition containing pioglitazone and glimepiride. Glimepiride which is one of components of the pharmaceutical composition disclosed by the invention can be used for lowering the blood sugar of a patient with II diabetes by stimulating insulin to release from a functional pancreatic beta cell. By virtue of a non-insulin mechanism, the glimepiride further can be used for increasing the sensitivity of a peripheral tissue to insulin. Another component pioglitazone hydrochloride is a peroxysome proliferator-activated receptor-gamma agonist with strong effect and high selectivity, and capable of lowering the insulin resistance of livers and the peripheral tissue, so that the insulin-dependent glucose utilization is increased, and the output of hepatic glucose is lowered.
Owner:SICHUAN HAISCO PHARMA CO LTD
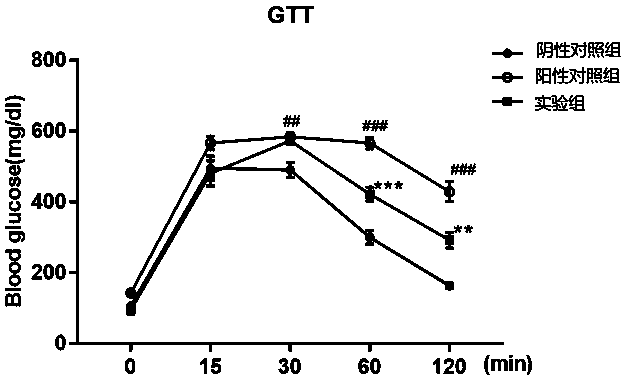
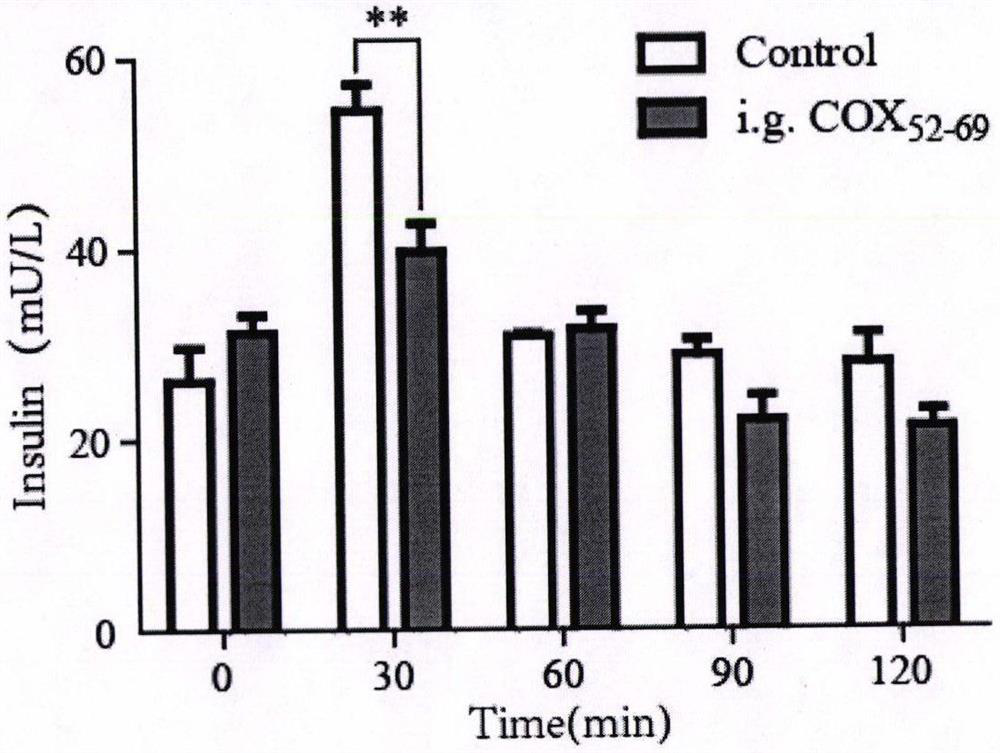
Cyclic peptide capable of reducing insulin and properly reducing blood sugar at same time
PendingCN113274483AImprove stabilityInhibition of secretionMetabolism disorderCyclic peptide ingredientsCyclic peptideLow insulin
The invention relates to a cyclic peptide capable of reducing insulin, not increasing blood sugar but properly reducing blood sugar at the same time, and belongs to the technical field of biological pharmacy. The bifunctional cyclic peptide is obtained through in-vitro modification on the basis of an original linear short peptide COX52-69; the effects of two seemingly contradictory aspects are achieved; namely, the effect of inhibiting insulin secretion but not increasing blood sugar at the same time is achieved; blood sugar is reduced; and the mechanism of the bifunctional cyclic peptide is possibly to improve the sensitivity of tissue to insulin. It is called as a bifunctional peptide. The bifunctional peptide can be used for congenital insulinemia, insulin tolerance, hyperinsulinemia of obese people and the like; literatures report that insulin increase is the reason of obesity; but people cannot lose weight through a method of reducing insulin all the time; and because blood sugar is increased after insulin is reduced, hyperglycemia is more adverse to health. The cyclic peptide realizes the function that the blood sugar is not increased but is reduced while insulin is reduced, so that a new medicine is developed for treating obesity; and the medicine not only can be administrated by injection, but also can be conveniently administrated by oral administration. The convenience of using the medicine is greatly improved.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
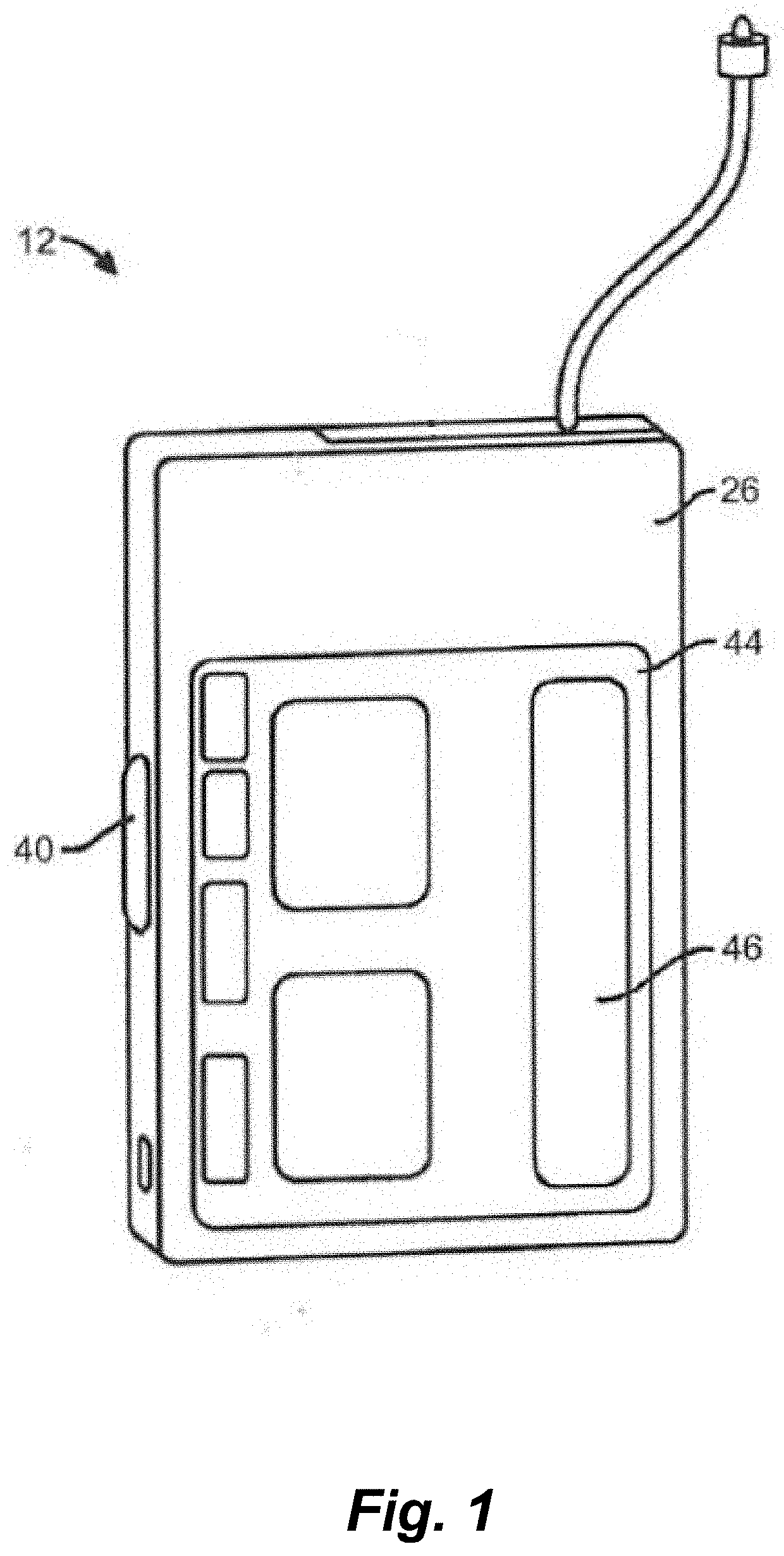
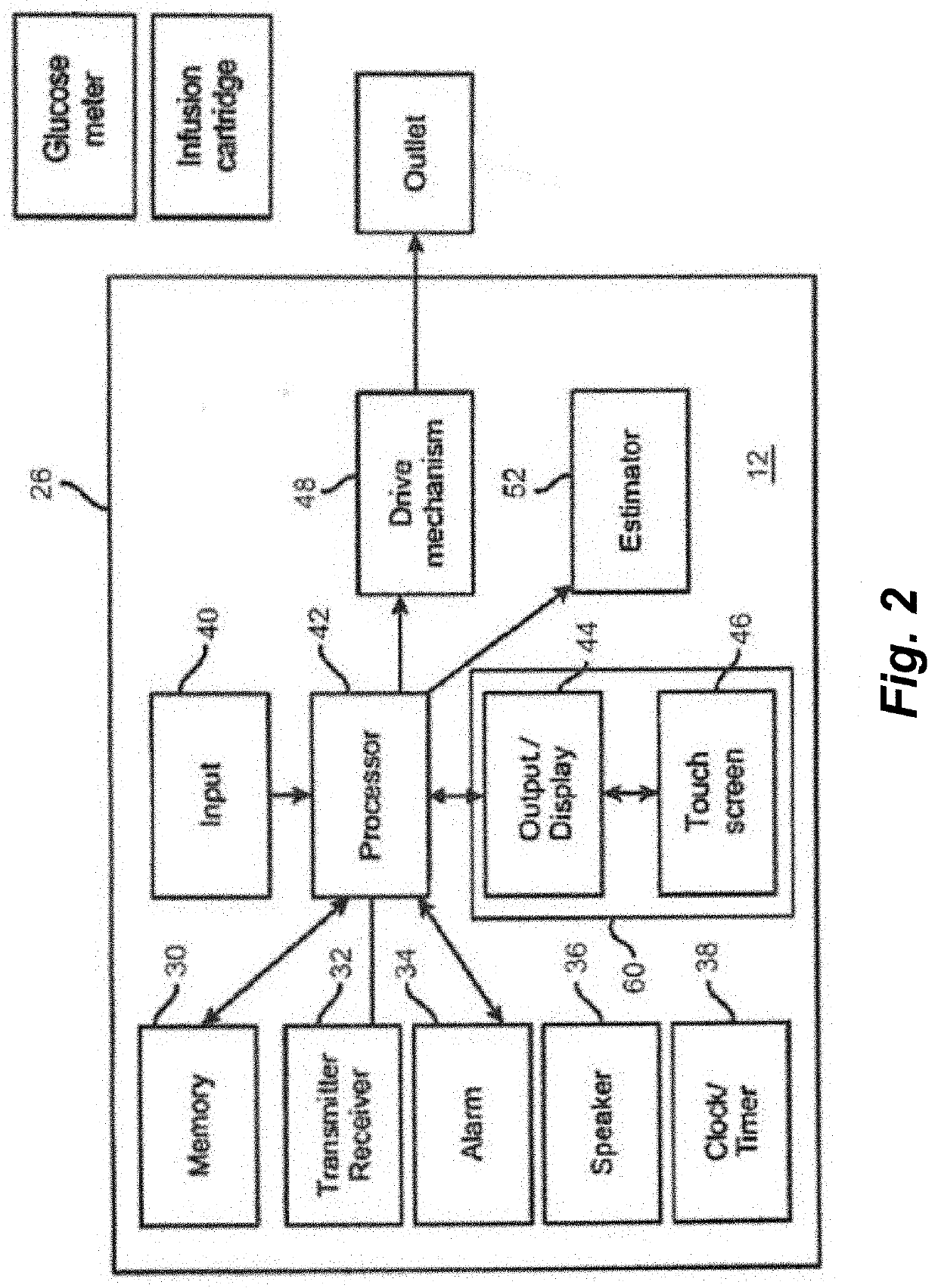
Systems and methods for automated insulin delivery response to meal announcements
PendingUS20210353857A1Increase insulin on board toleranceAvoid oscillationDrug and medicationsMedical devicesPhysiologyBasal rate
Disclosed herein are apparatuses and methods that account for meal announcements in closed loop insulin delivery systems. Rather than simply increasing an insulin delivery rate in response to the meal announcement, the closed loop algorithm can be modified to increase the insulin on board tolerance during eating periods. This approach utilizes the stability of the cascaded loop in the closed loop algorithm to prevent the oscillations in glucose levels that can occur by simply increasing the basal rate.
Owner:TANDEM DIABETES CARE INC
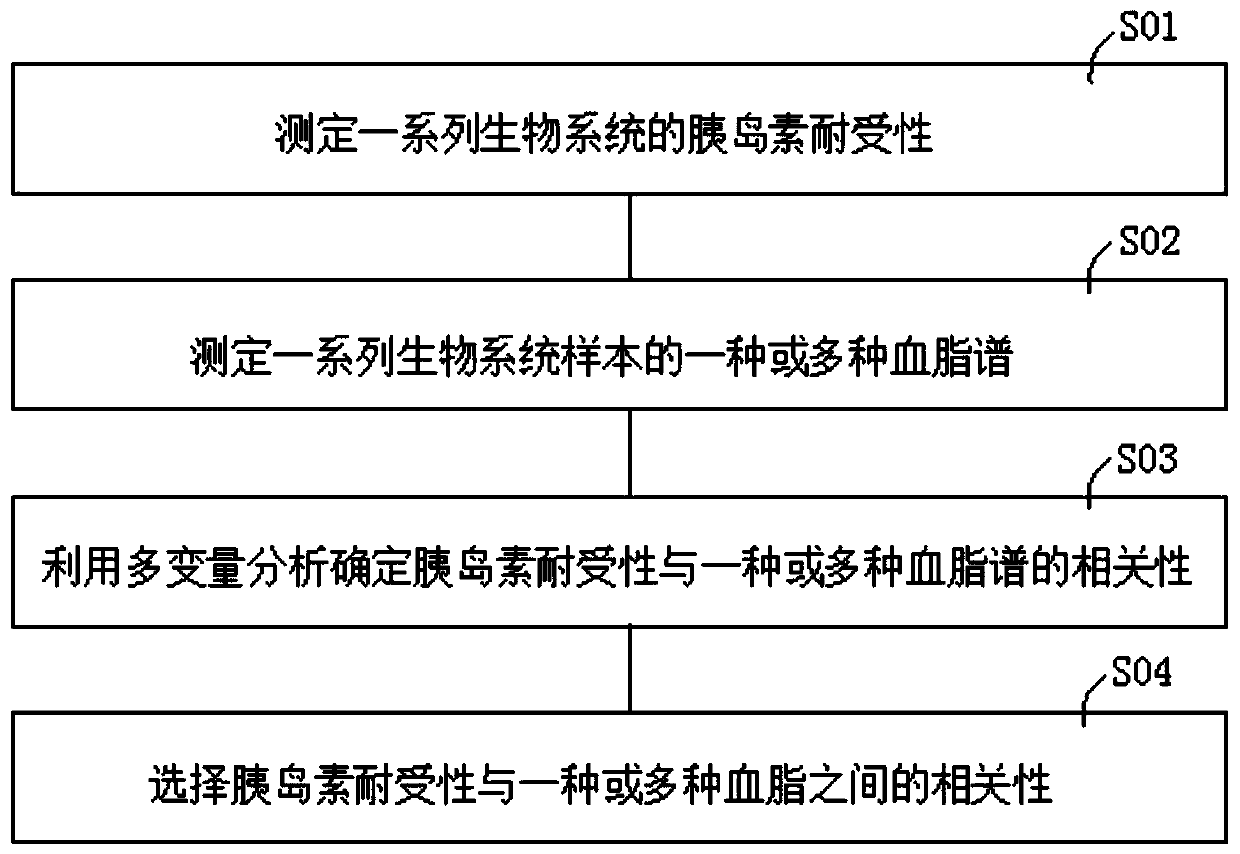
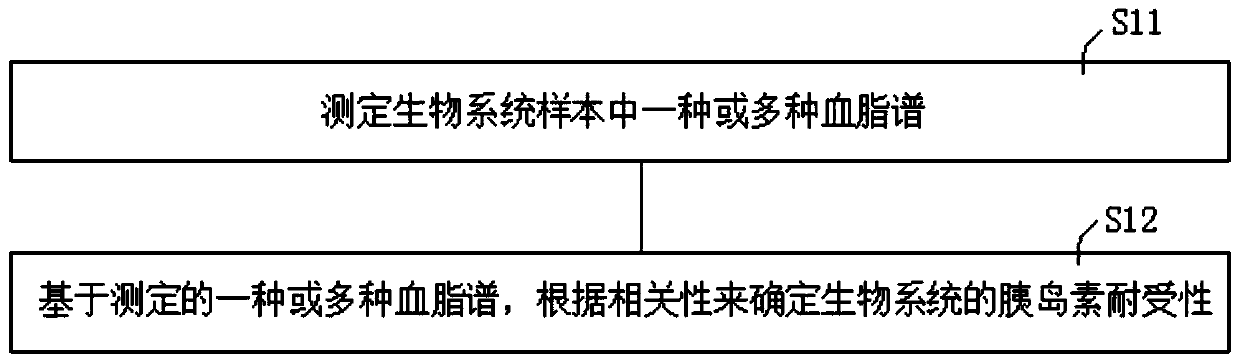
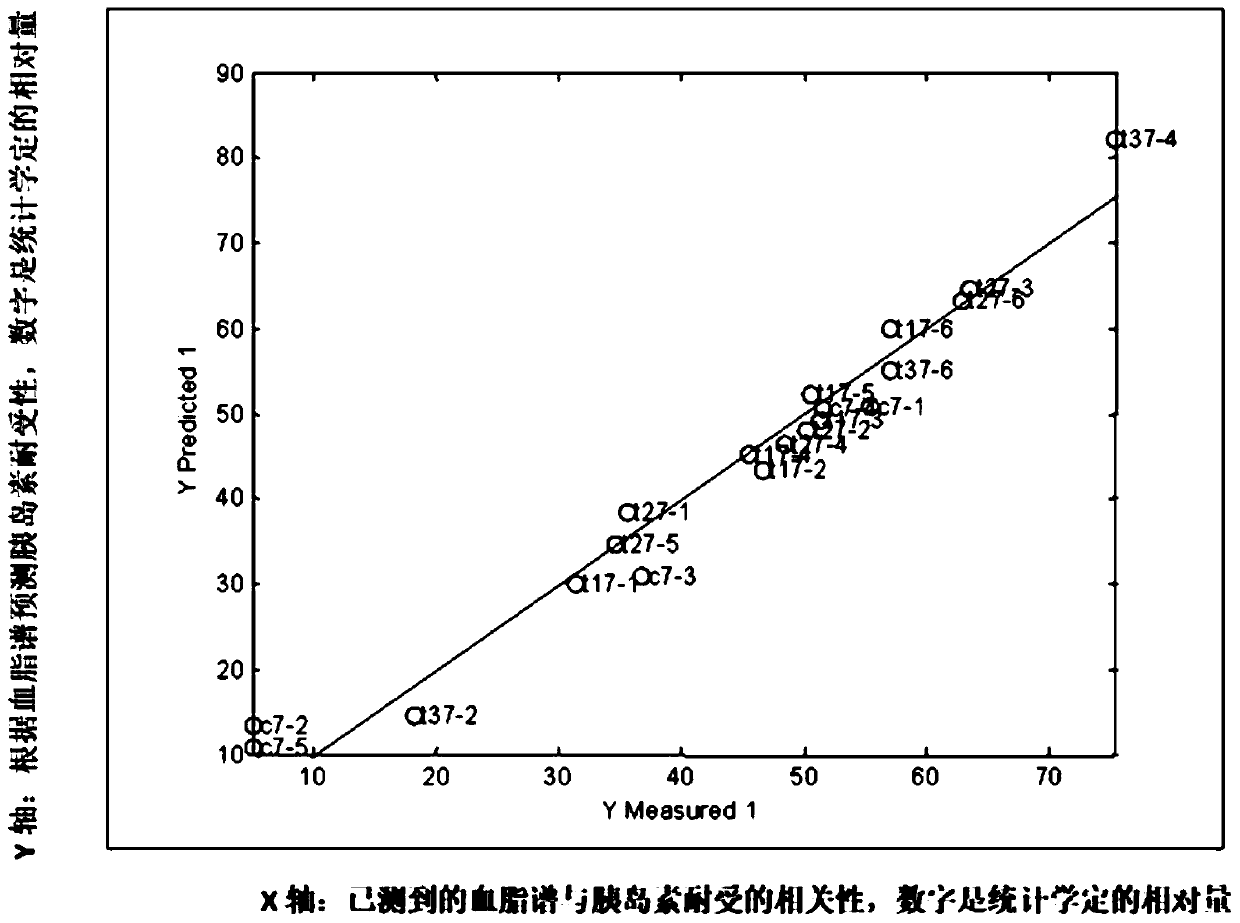
Method of determining blood lipid profile associated with insulin tolerance and application and device thereof
The present invention relates to a method of determining a blood lipid profile associated with insulin tolerance. The method comprising the steps: determining insulin tolerance of a series of biological systems; determining one or more blood lipid profiles of a series of biological system samples; utilizing multivariable analysis to determine the correlation between insulin tolerance and one or more blood lipid profiles; and selecting the correlation between insulin tolerance and one or more blood lipids. According to the method, the correlation between one or more blood lipid profiles of thebiological system and the insulin tolerance of the biological system is determined through the multivariable analysis method, the insulin tolerance of the biological system can be determined accordingto the detection condition of blood lipid in practical application according to the correlation and the method is simple and low in cost.
Owner:SHENZHEN KAIWUCHENGWU TCM TECH CO LTD
Remedy for diabetes
The object of the present invention is to provide a therapeutic agent for diabetes, an agent for improving insulin resistance, or a food and beverage, a food and beverage additive, feed or a feed additive for treating diabetes or improving insulin resistance. In order to solve this problem, the present invention provides a therapeutic agent for diabetes containing hydroxyproline, a hydroxyproline derivative, or a pharmacologically acceptable salt thereof as an active ingredient, and an insulin resistance improving agent for use in the treatment of diabetes or Food and drink, food and drink additives, feed and feed additives for insulin resistance improvement.
Owner:KYOWA HAKKO KOGYO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com