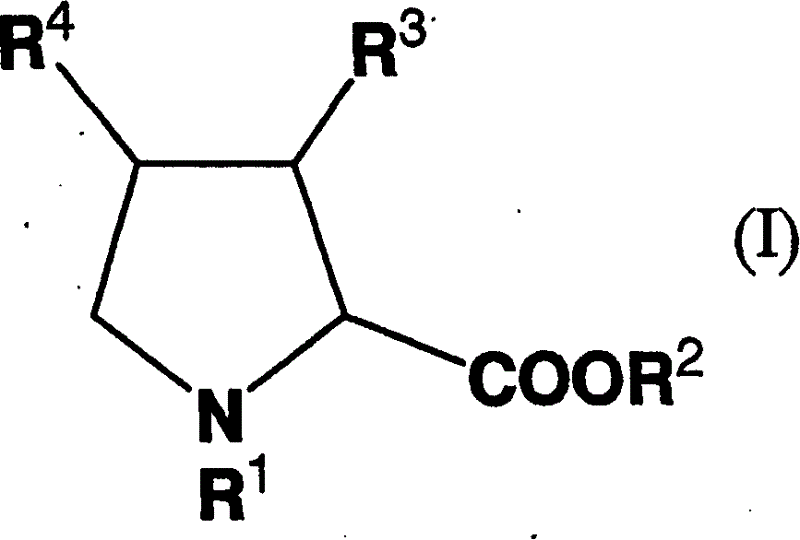
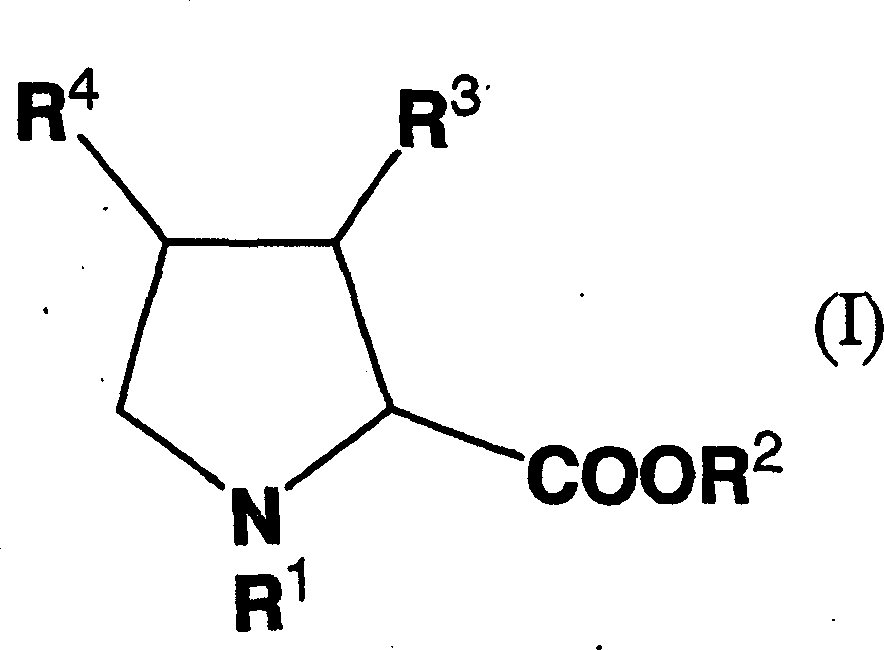
Remedy for diabetes
A technology for diabetes and therapeutic agents, applied in the field of feed and feed additives, which can solve the problems of no reports of improving insulin resistance, no indication of effectiveness of hydroxyproline and hydroxyproline derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Fifteen type II diabetes animal model KK-Ay / Ta Jcl mice (CREA, Japan, male, 6 weeks old) were divided into 3 groups with 5 mice in each group, which were designated as the first group to the third group.
[0082] The mice in groups 1 to 3 were allowed to eat and drink freely. The mice in the first group were given a commercially available feed CE-2 (manufactured by CREA Japan). The mice of the second group ingested CE-2 supplemented with 1% by weight of trans-4-hydroxy-L-proline (manufactured by Kyowa Hakko Kogyo Co., Ltd., hereinafter abbreviated as hydroxyproline). The mice in the third group ingested CE-2 supplemented with 1% by weight of trans-N-acetyl-4-hydroxy-L-proline (manufactured by Kyowa Hakko Kogyo Co., Ltd., hereinafter abbreviated as N-acetylhydroxyproline) .
[0083] Blood was collected from the tail vein on the first day and 17 days after the start of the experiment, and the blood glucose level without fasting was measured with MEDISAFE leaderGR-101 (m...
Embodiment 2
[0094] Fifteen type II diabetes animal model KK-Ay / Ta Jcl mice (CREA, Japan, male, 6 weeks old) were divided into 3 groups with 5 mice in each group, which were designated as the first group to the third group.
[0095] The mice in groups 1 to 3 were allowed to eat and drink freely. The mice in the first group were given a commercially available feed CE-2 (manufactured by CREA Japan). The mice of the second group ingested CE-2 supplemented with 1% by weight of trans-4-hydroxy-L-proline ethyl ester (manufactured by Sanyo Chemical Laboratories, hereinafter abbreviated as hydroxyproline ethyl ester). Let the mice in the third group ingest trans-N, O-diacetyl-4-hydroxy-L-proline oleyl ester (manufactured by Nippon Chemical Corporation, hereinafter abbreviated as diacetyl hydroxyproline oil) added with 1% by weight. Enyl esters) CE-2.
[0096] On the first day of the experiment and after the 10th day, blood was collected from the tail vein, and the blood glucose value without fas...
Embodiment 3
[0106] Sixteen type II diabetes animal model KK-Ay / Ta Jcl mice (CREA, Japan, male, 6 weeks old) were divided into 2 groups of 8 mice each, designated as Group 1 and Group 2.
[0107] After an 18-hour fast, physiological saline was orally administered to the mice of Group 1, and 20% (w / v) Aqueous solution of N-acetylhydroxyproline (dissolved in physiological saline). One hour later, 40% (w / v) glucose aqueous solution was orally administered to the mice of the first group and the second group at 2 g / kg body weight B.W. to load them with glucose. Blood was collected from the tail vein 60 minutes before (-60 minutes), at the time of administration (0 minutes), 30 minutes after administration, and 120 minutes after administration of the glucose solution, and blood glucose levels were measured using MEDISAFE leader GR-101. Values are represented by mean±standard error (n=8), and statistical reliability (p value) is calculated by t test.
[0108] The measurement results of the b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com